






























cat no | io1021
ioMicroglia Male
Male human iPSC donor-derived microglia
-
Cryopreserved human iPSC-derived cells powered by opti-ox, that are ready for functional experiments in 4 days
-
Ideal for in vitro multi-cellular neuroinflammation studies and neurodegenerative disease modeling
-
Consistently perform key phagocytic and cytokine secretion functions, and are co-culture compatible

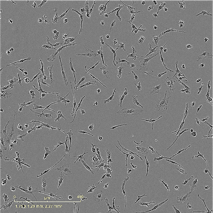
Human iPSC-derived microglia, male donor

ioMicroglia readily phagocytose bioparticles by day 4 post-revival
Day 4, 6 and 10 ioMicroglia Male were incubated with 1 µg/0.33 cm2 pHrodo RED labelled E. coli particles for 24 hour. Images were acquired every 30 mins on the Incucyte looking at red fluorescence and phase contrast. The graphs display the proportion of cells phagocytosing (left), and the fluorescence intensity per cell displaying degree of phagocytosis (right). Three technical replicates were performed. Seeding density 60,000 cells/cm2.
Cells readily phagocytose by day 4 post-revival in a similar response as day 10, providing a experimental window to perform this key assay.

Phagocytosis of Amyloidβ-42 particles by male donor-derived ioMicroglia
Day 10 male donor-derived ioMicroglia were incubated with 500 nM AF488 labelled Amyloidβ-42 (AnaSpec) for 24 hours with images acquired every 30 mins on the Incucyte. Seeding density 60,000 cells/cm2. Three technical replicates were performed.

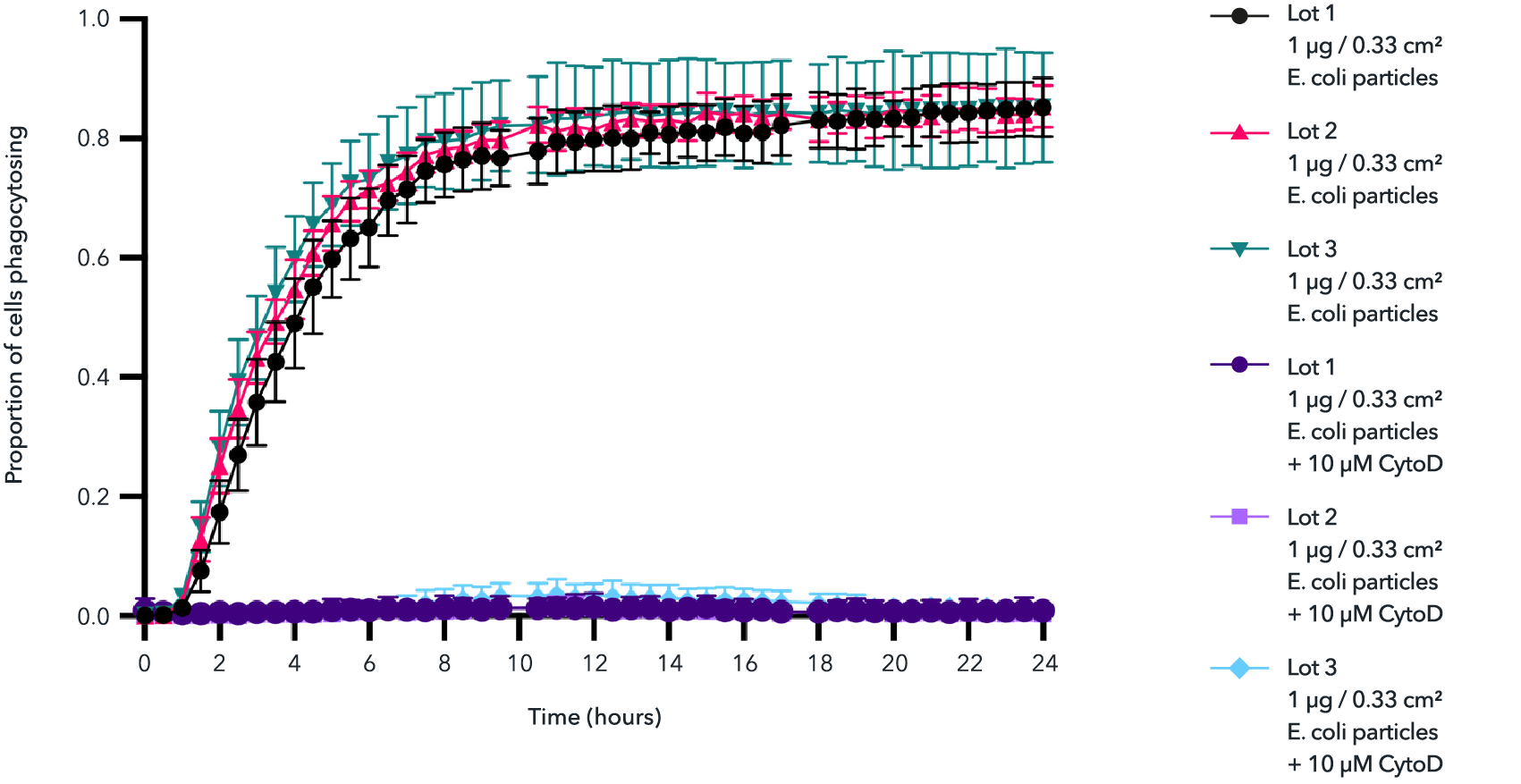
High lot-to-lot consistency in phagocytosis of E. coli particles by male donor-derived ioMicroglia
Day 10 male donor-derived ioMicroglia from three independent lots were incubated with 1 µg/0.33 cm2 pHrodo RED labelled E. coli particles for 24 hours +/- cytochalasin D control. Images were acquired every 30 mins on the Incucyte® looking at red fluorescence and phase contrast. The graph displays the proportion of cells phagocytosing E. coli particles over 24 hours. Three technical replicates were performed per lot.

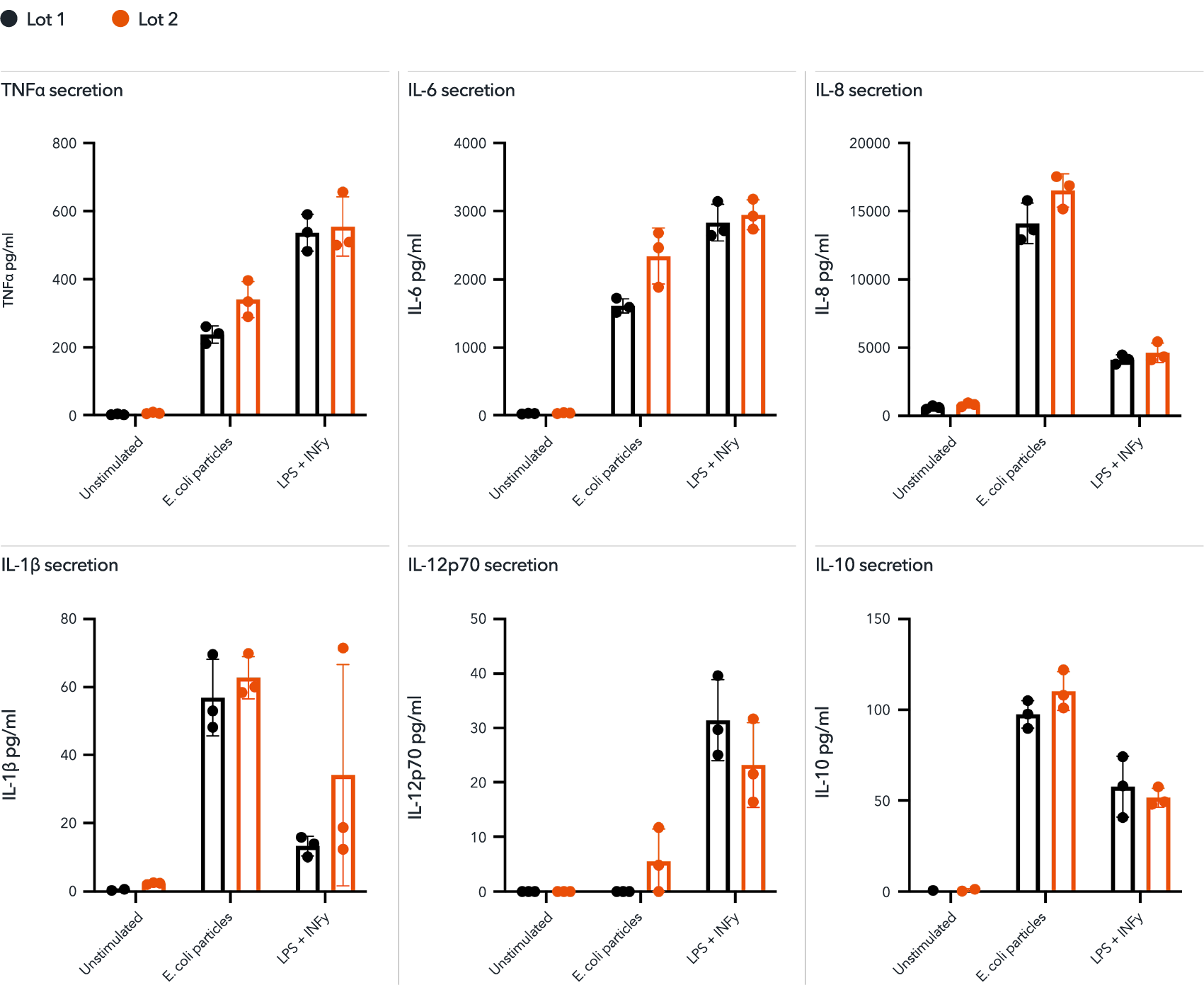
Male donor-derived ioMicroglia secrete pro-inflammatory cytokines upon activation
Day 10 male donor-derived ioMicroglia were stimulated with LPS 100 ng/ml and IFNɣ 20 ng/ml for 24 hours or pHrodo RED labelled E. coli particles. Supernatants were harvested and analysed using MSD V-plex Proinflammatory Kit. These cells secrete TNF⍺, IL-6, IL-8, IL-1b, IL-12p70 and IL-10 in response to stimuli. Predominantly producing a pro-inflammatory response. This is consistent across two independent lots. Three technical replicates were performed per lot.
View the cytokine release protocol used to generate this data.

ioMicroglia show a pro-inflammatory cytokine response to Amyloidβ-42 stimulation
Day 10 male donor-derived ioMicroglia and female donor-derived ioMicroglia were stimulated for 24 hours with LPS (100 ng/ml) and IFNɣ (20 ng/ml), or synthetic Amyloidβ-42 oligomers (10 μM, StressMarq). Supernatants were harvested after 24 hours and analysed with using the MSD V-PLEX Proinflammatory Kit. Secretion levels were normalised to cell count per field of view (FOV) to account for variations in cell density. Seeding density 80,000 cells/cm2.
A clear response to Amyloidβ-42 is seen in both cell types, with background specific differences also observed.
View the cytokine release protocol used to generate this data.

ioMicroglia demonstrate a measurable pro-inflammatory cytokine response by day 3
Day 3, 7, and 10 male donor-derived ioMicroglia were stimulated with LPS (100 ng/ml) and IFNɣ (20 ng/ml) for 24 hours, and supernatants and analysed using the MSD V-PLEX Proinflammatory Kit. Bars represent 3 technical replicates with standard error of mean.
A clear response is seen by day 3 post-revival, with the highest secretion levels observed at 10, demonstrating a wide time frame to perform this key assay.
View the cytokine release protocol used to generate this data.

ioMicroglia enhance network activity in co-culture with ioGlutamatergic Neurons
ioGlutamatergic Neurons expressing Incucyte Neuroburst Orange Lentivirus mono-culture or in co-culture with ioMicroglia Male monitored and quantified using Incucyte Neuronal Activity Analysis software.
A) Representative calcium traces shown for each culture condition at 15 days post-microglia addition.
B) Bar charts at 15 days post-microglia addition showing network correlation and mean burst duration. Data presented as mean ± SEM, n = 3 – 12 replicates.
View the co-culture protocol used to generate this data.
This data was generated by Jasmine Trigg and colleagues at Sartoirus, taken from the application note: "Advanced in vitro Modeling of Human iPSC-derived Neuronal Mono- and Co-cultures with Microglia: Optimization Using Growth Factors and Live-Cell Analysis".

Flow cytometry analysis of male donor-derived ioMicroglia shows key phenotypic marker expression
Flow cytometry analysis of day 10 male donor-derived ioMicroglia shows key microglia marker expression of P2RY12, CD14, CD45 and CD11b with a purity of above 95%.
opti-ox precision deterministically programmed ioMicroglia from a male donor rapidly form a homogenous microglia population
Time-lapse video capturing the rapid and homogeneous microglia phenotype acquisition upon thawing of cryopreserved male donor-derived ioMicroglia. 10 day time course.

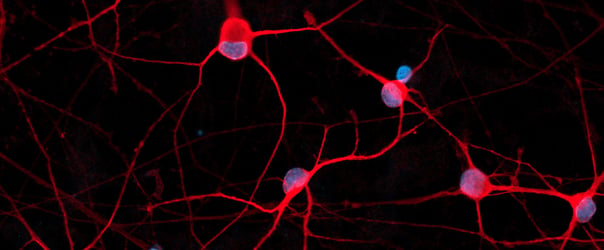
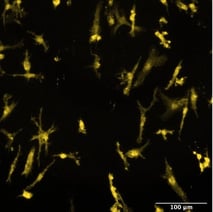
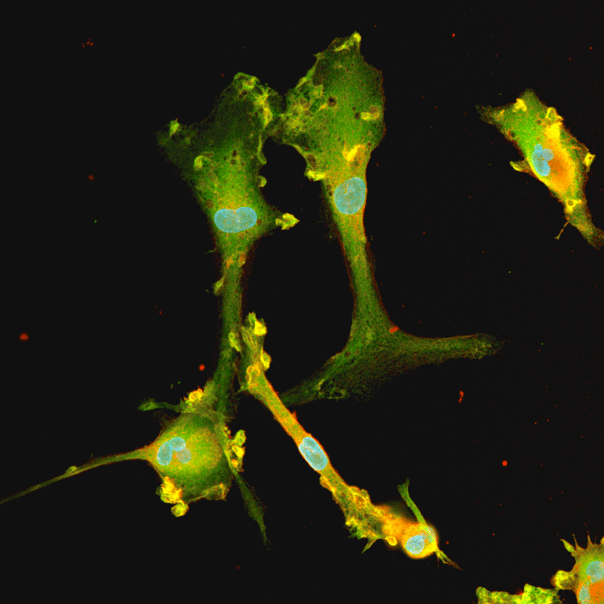
Male donor-derived ioMicroglia show key microglia marker expression
Immunofluorescent staining of day 10 male donor-derived ioMicroglia shows homogenous expression of IBA1 and TREM2, and a typical ramified morphology. DAPI counterstain (blue). Image taken at 10x magnification.

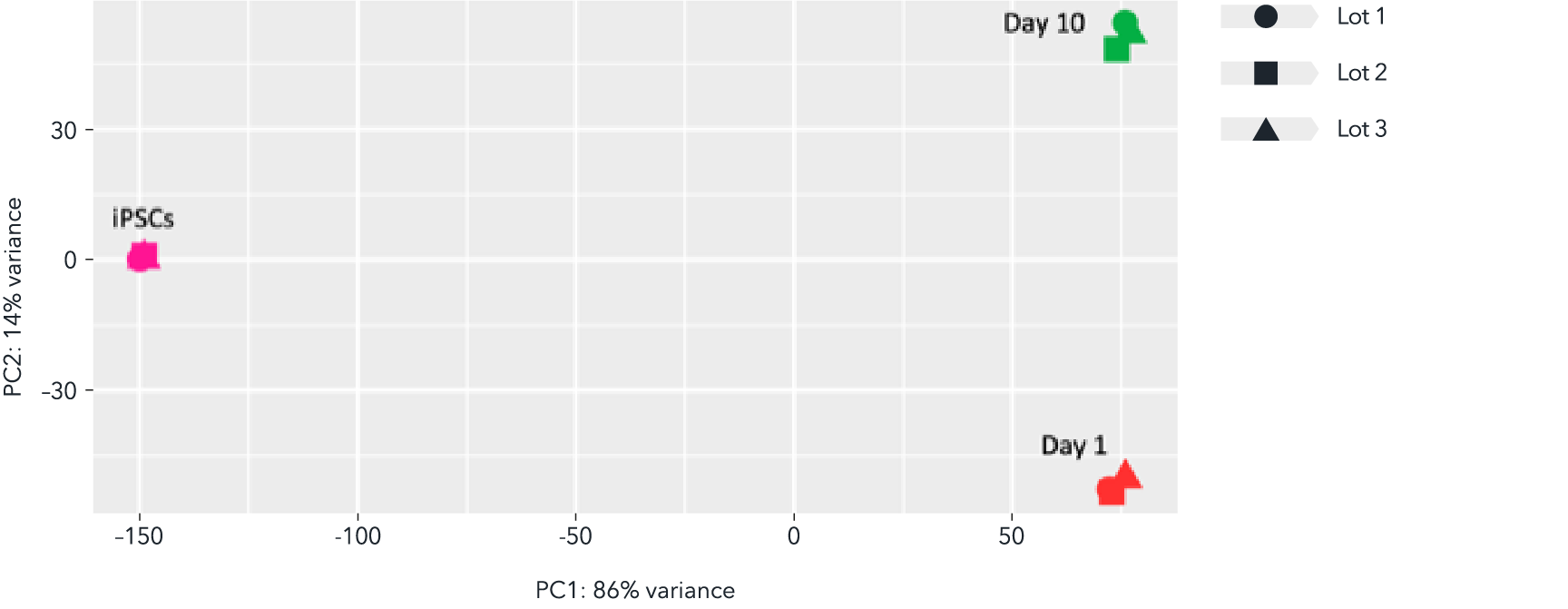
Whole transcriptome analysis demonstrates high lot-to-lot consistency of male donor-derived ioMicroglia
Bulk RNA sequencing analysis was performed on three independent lots of male donor-derived ioMicroglia at three different time points throughout the reprogramming protocol. Principal component analysis represents the variance in gene expression between the lots and shows the high consistency across each lot at each given time point. Populations of male donor-derived ioMicroglia with equivalent expression profiles can be generated consistently from every vial, allowing confidence in experimental reproducibility.

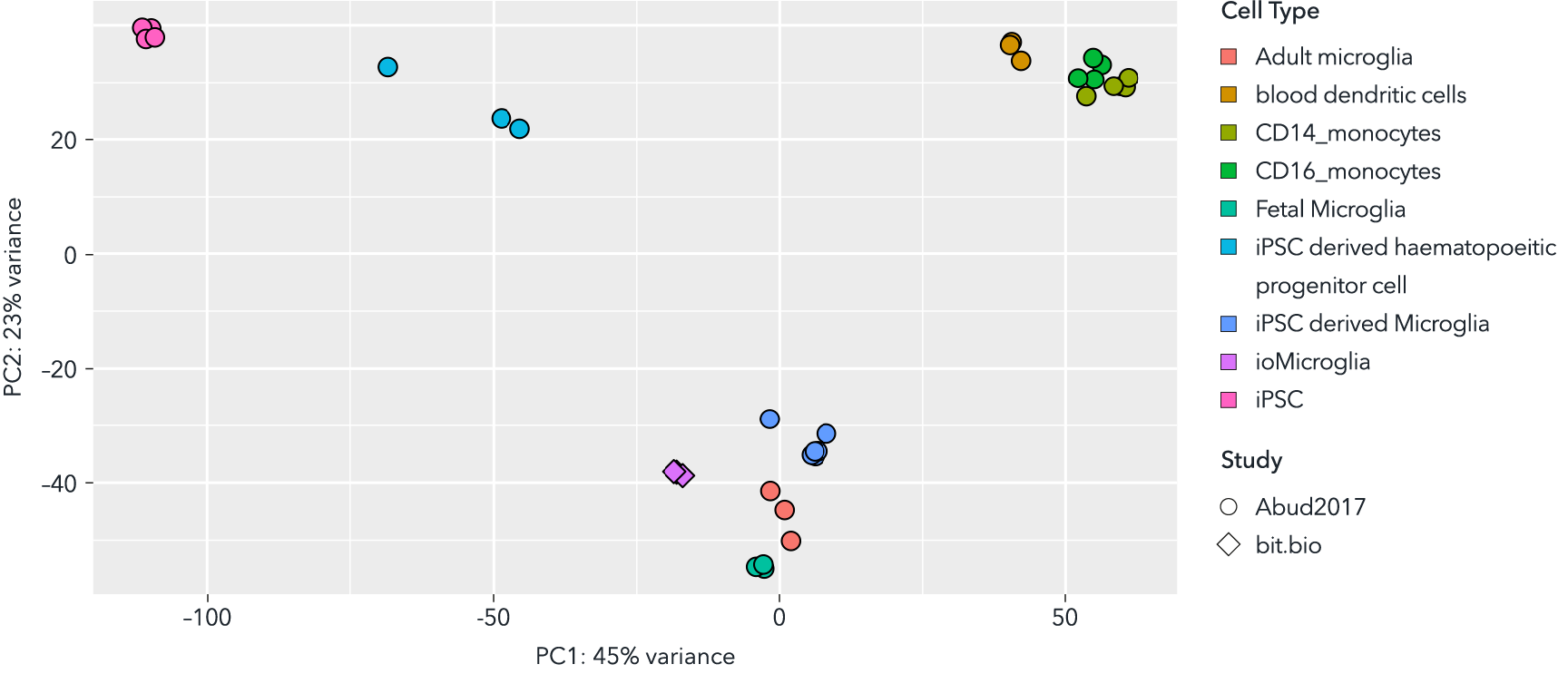
Whole transcriptome analysis demonstrates that male donor-derived ioMicroglia are highly similar to primary adult, foetal and other iPSC-derived microglia
Principal component analysis of bulk RNA sequencing data from male donor-derived ioMicroglia, integrated with sequencing data from Abud et al. (1) shows that these cells cluster closely to primary foetal and adult microglia data sets derived from this publication. Shapes represent the experiment from which data was obtained and colours represent the cell type.
(1) Abud E, et al., Neuron, 2018; 94(2): 278-293

Male donor-derived ioMicroglia display C5a-mediated chemotaxis in a dose-dependent manner
Male donor-derived ioMicroglia were seeded and matured in a plate for 1 week before adding C5a (chemoattractant) and scanning for cell migration. The chemotaxis assay was performed using Clearview plates with the Incucyte Chemotaxis Analysis Software module. A and B, confluency in the insert (top area) decreased over time as the cells migrated towards C5a, via pores in the insert into a reservoir containing C5a, in a dose-dependent manner. Corresponding confluency in the reservoir (bottom area) increased over time. C and D, Maximal migration of cells is observed at 10 nM with a 5-fold increase in cells present in the reservoir at 60 hours. Explore the full dataset.
This data was generated by Eve Corrie and Emma V. Jones from Medicines Discovery Catapult.
Eve Corrie, Emma V. Jones, Medicines Discovery Catapult, Block 35, Mereside, Alderley Park, Macclesfield, SK10 4ZF. UK.
Male donor-derived ioMicroglia form co-cultures with ioGlutamatergic Neurons
ioGlutamatergic Neurons (io1001) were cultured to day 10 post-thaw. Male donor-derived ioMicroglia (io1021) cultured to either day 1 or day 10 post-thaw were added directly to day 10 ioGlutamatergic Neurons. The co-cultures were maintained for a further 8 days. Imaging was performed in 30-minute intervals. Representative video showing that these cells form a stable co-culture with ioGlutamatergic Neurons.

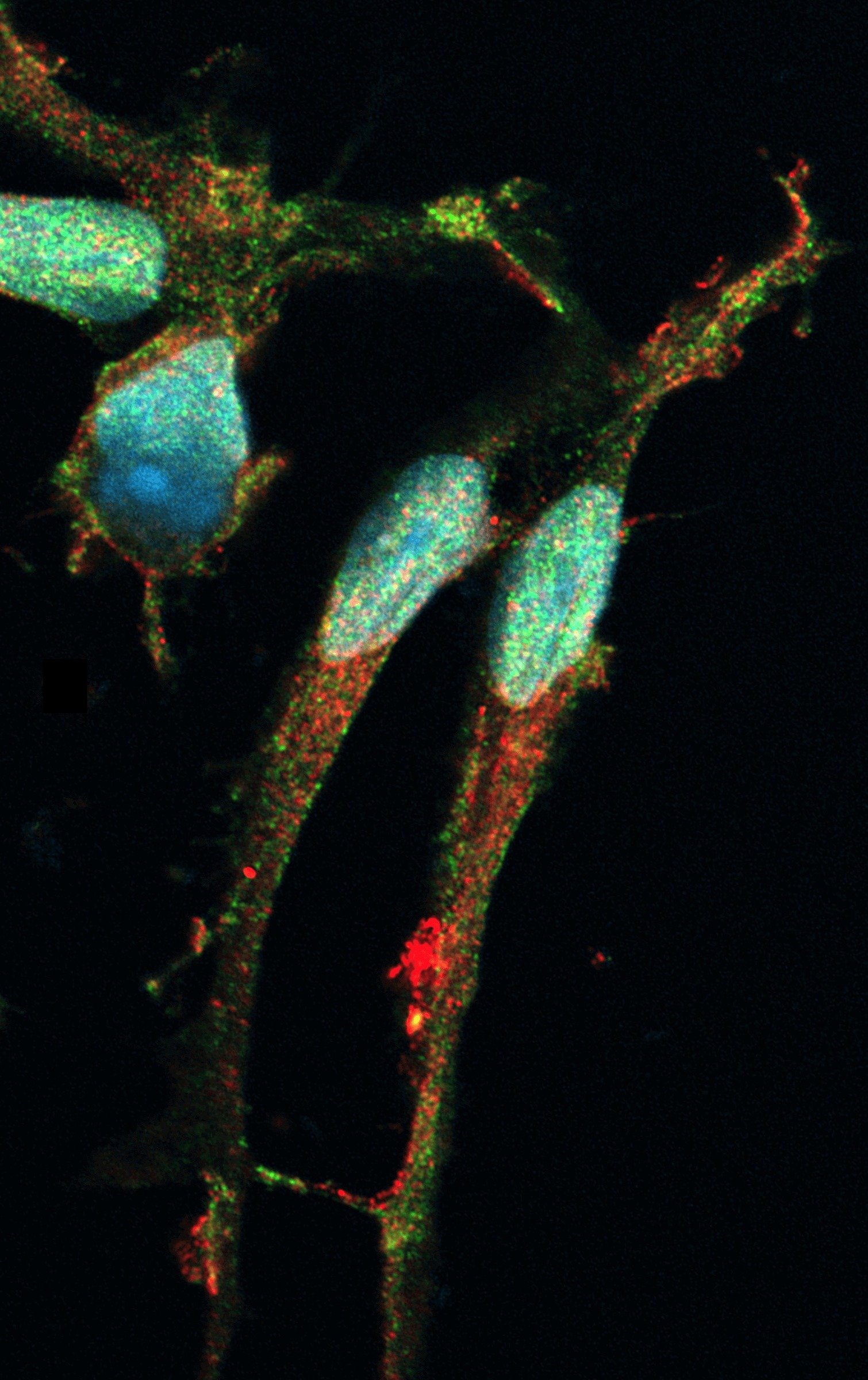
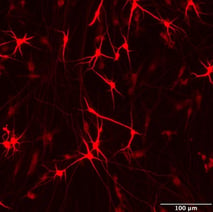
Co-cultures of ioMicroglia and ioGlutamatergic Neuron show key marker expression
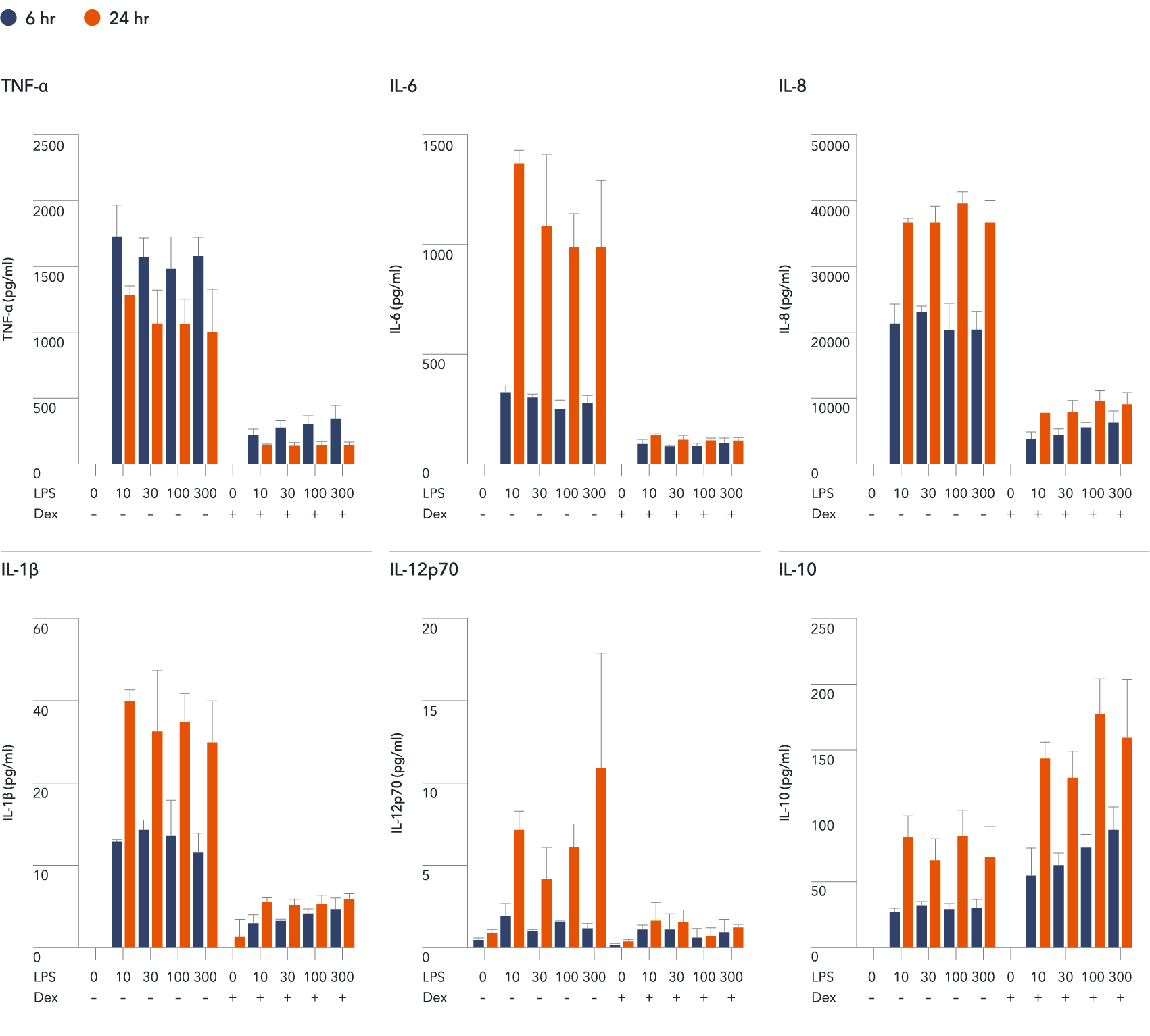
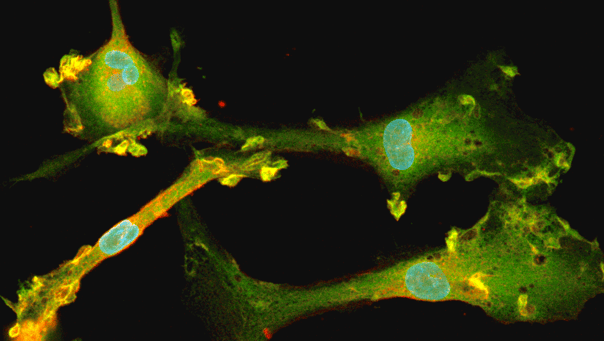
Immunofluorescent analysis at day 8 of the co-cultures shows expression of the microglia marker, IBA1 (yellow) and the pan-neuronal marker, MAP2 (red), as expected. Representative images taken at 10X magnification with 100 μm scale bar.
View the co-culture protocol used to generate this data.Phagocytic function is retained in co-culture with ioGlutamatergic Neurons
Representative video showing male donor-derived ioMicroglia in co-culture with ioGlutamatergic Neurons selectively phagocytosing pHrodo Red labelled Zymosan particles, without any adverse effects on neuron morphology. ioMicroglia start to fluoresce red when they have engulfed material which is initiated by a shift in pH.

Live-cell imaging reveals clear visualisation of GFP ioMicroglia when co-cultured with ioGlutamatergic Neurons
ioGlutamatergic Neurons (io1001) were cultured to day 10 post-thaw. GFP ioMicroglia (io1096) were cultured to day 10 post-thaw and were directly added to day 11 ioGlutamatergic Neurons. The co-cultures were maintained for a further 3 days before live-cell imaging with Leica DMi8. Brightfield and fluorescence images were taken and merged, easily demonstrating distribution of GFP ioMicroglia within the co-culture.
View the co-culture protocol used to generate this data.
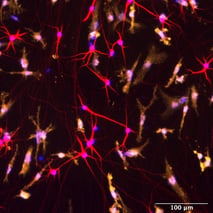
Quad-culture model with ioMicroglia, ioOligodendrocyte-like cells, ioGlutamatergic Neurons, and human iPSC-derived astrocytes
Complex multi-cellular models have the potential to provide insights into the role of glial cells in disease mechanisms of neurodegenerative diseases, such as Alzheimer’s disease and multiple sclerosis.
Immunofluorescent staining of a multi-cellular culture including ioOligodendrocyte-like cells expressing MBP (green), ioGlutamatergic Neurons expressing NF-200 (red) and TUBB3 (red), ioMicroglia expressing IBA1 (yellow), human iPSC-derived astrocytes expressing S100B (yellow) and GFAP (green), and DAPI counterstain (blue). Cultures were analysed on day 14.
Data courtesy of Bsibsi, M. et al., 2024, Charles River Laboratories, presented in a poster at the Society of Neuroscience 2024 meeting.

ioMicroglia are efficiently transfected with mRNA encoding GFP
ioMicroglia Male are efficiently transfected and show sustained long-term expression of mRNA encoding GFP. Cells were imaged throughout the experiment to assess transfection efficiency and evaluate potential cytotoxic effects of the transfection protocol. Day 4 images were captured prior to transfections on the same day.
Download the step-by-step protocol for lipid-based delivery of synthetic mRNA into ioMicroglia.
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.











Hoescht(blue)TUBB3(blue)_day4.png?width=604&name=bit.bio_ioGlutamatergic%20Neurons_60xMAP2(red)Hoescht(blue)TUBB3(blue)_day4.png)


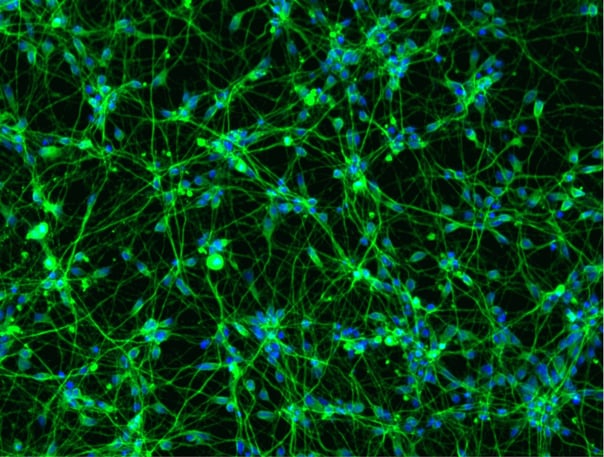




_MAP2(R)_Tubb3(B)_Hoechst(B)_20x_merge-comp.jpg?width=604&name=Colour%20webinar%20with%20it-bio%20ioGlutamatergic%20Neurons_VGLUT2(G)_MAP2(R)_Tubb3(B)_Hoechst(B)_20x_merge-comp.jpg)
Hoescht(blue)_day12v2.png?width=604&name=bit.bio_ioGlutamatergic%20Neurons_20xMAP2(red)Hoescht(blue)_day12v2.png)