cat no | io1103 Early Access
CRISPRi-Ready ioMicroglia Male
Male human iPSC donor-derived microglia expressing modified dCas9 for gene repressions and CRISPRi screens
-
Cryopreserved human iPSC-derived cells powered by opti-ox, that are ready for experiments in days
-
Microglia constitutively expressing modified dCas9 optimised for gene repressions and CRISPRi screens
-
Perform key phagocytic and cytokine secretion functions, and are co-culture compatible

Male human iPSC donor-derived microglia expressing modified dCas9 for gene repressions and CRISPRi screens

Flow cytometry analysis demonstrates gene repression of B2M upon lentiviral gRNA delivery
Flow cytometry analysis confirmed robust B2M gene repression in CRISPRi-Ready ioMicroglia following lentiviral delivery of a B2M-targeting gRNA on day 10 post-thaw using VPx-VLP for enhanced lentiviral gRNA delivery. Gene repression was assessed 5 days later.
(A) 32% of cells were GFP+ indicating successful delivery of the B2M-targeting gRNA, with 72% of these GFP+ cells showing robust repression.
(B) The dCas9 transcriptional repressor induced a 5.7-fold increase in B2M protein expression (blue) relative to non-targeting control (grey), as measured by geometric mean fluorescence intensity (GMFI) in the GFP+ population.

Immunocytochemistry analysis demonstrates gene repression of IBA1 upon lentiviral gRNA delivery in a 96-well plate
IBA1 targeting gRNAs were introduced into CRISPRi-Ready ioMicroglia at day 10 post-thaw via lentiviral transduction in a 96-well plate format, using VPx-VLP for enhanced lentiviral gRNA delivery. After 5 days, transduction and IBA1 repression efficiencies were quantified by immunocytochemistry. Comparable results were observed in 24-well plates.
(A) Representative images showing repression of IBA1 in a majority of cells.
(B) Quantification of IBA1 positive cells reveals >75% inhibition efficiency.

CRISPRi-Ready ioMicroglia show ramified morphology by day 4
CRISPRi-Ready ioMicroglia mature rapidly and key ramified morphology can be identified by day 4 and continues through to day 10, similar to ioMicroglia Male (io1021). Day 1 to 10 post-thawing; 100x magnification.

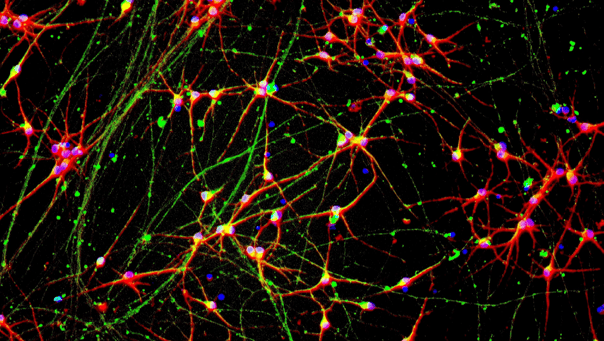
CRISPRi-Ready ioMicroglia homogeneously express IBA1
Immunofluorescent staining on day 10 post-revival demonstrates homogenous expression of key microglia marker IBA1 and ramified morphology in CRISPRi-Ready ioMicroglia, similar to ioMicroglia Male (io1021). 100x magnification.

Phagocytosis of E. coli particles by CRISPRi-Ready ioMicroglia
Phagocytosis assay using pHrodo E. coli BioParticles at day 10 post-thaw demonstrates efficient uptake of bioparticles by CRISPRi-Ready ioMicroglia, in a similar manner to ioMicroglia Male (io1021) over 24 h. The graphs display the proportion of cells phagocytosing (left), and the fluorescence intensity per cell displaying degree of phagocytosis (right). The addition of Cytochalasin D (CytoD), an inhibitor of actin polymerisation, significantly decreased E.coli particle uptake as expected.

CRISPRi-Ready ioMicroglia secrete pro-inflammatory cytokines upon activation
CRISPRi-Ready ioMicroglia were stimulated at day 10 post-thaw with LPS 100 ng/mL and IFNɣ 20 ng/mL for 24 hours. Cell culture supernatants were collected and cytokine secretion levels were quantified by ELISA. Upon activation, these cells secreted TNF-α and IL-6 at comparable levels to wild-type ioMicroglia Male (io1021).
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.







 Emmanouil Metzakopian
Emmanouil Metzakopian