cat no | io1107 Early Access
GFP ioGlutamatergic Neurons
Human iPSC-derived glutamatergic neurons constitutively expressing GFP
- Cryopreserved human iPSC-derived cells powered by opti-ox that are ready for experiments in days
- Glutamatergic neurons constitutively expressing GFP, easy to track in multi-cell cultures, ideal for live-cell imaging
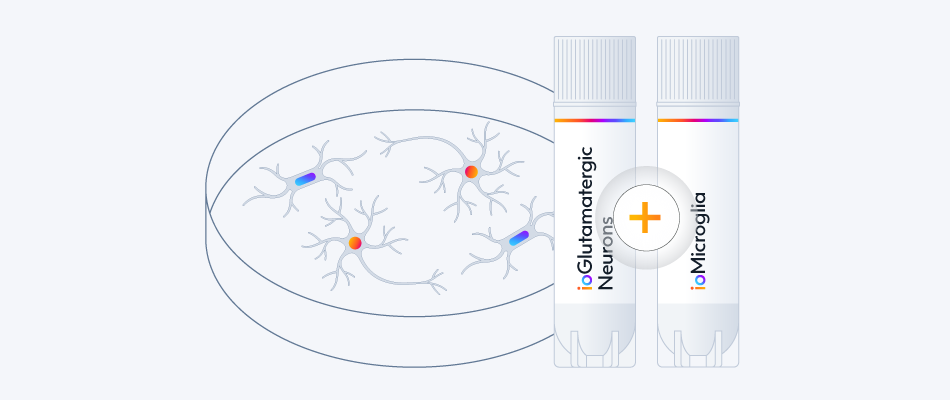
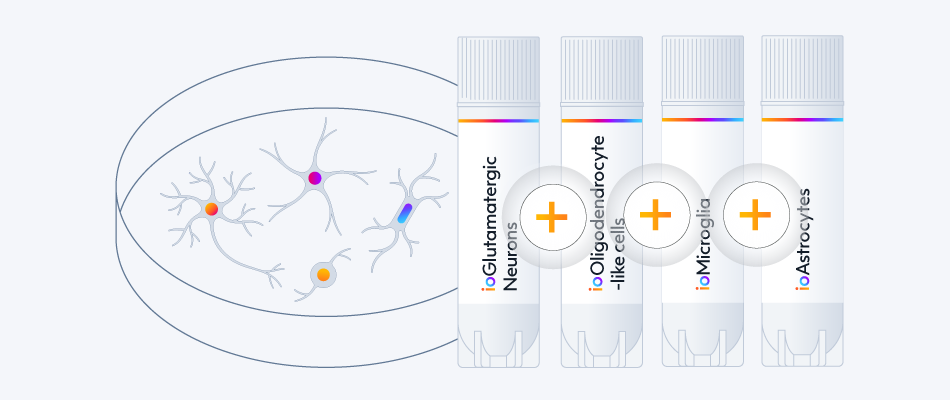
- Co-culture compatible with ioMicroglia and ioAstrocytes
Human iPSC-derived glutamatergic neurons constitutively expressing GFP
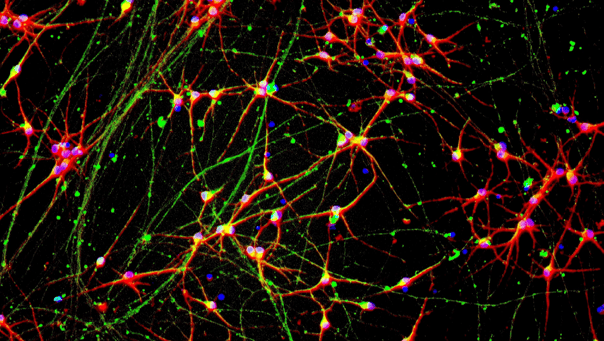
GFP ioGlutamatergic Neurons express key pan-neuronal and glutamatergic-specific markers
Immunofluorescent staining 11 days post-revival shows similar homogenous expression of pan-neuronal markers MAP2 and TUBB3 (upper panel) and the glutamatergic transporter VGLUT2 (lower panel) in both GFP ioGlutamatergic Neurons and the wild-type control.
The GFP signal is visible exclusively in GFP ioGlutamatergic Neurons and absent in the wild-type control (lower panel). 10X magnification.
Flow cytometry analysis of GFP expression in GFP ioGlutamatergic Neurons at day 11 and day 21
Flow cytometry analysis demonstrating GFP expression in >99% of cells for GFP ioGlutamatergic Neurons cultured until day 11 (centre), and no GFP expression seen in wild-type ioGlutamatergic Neurons (left). At day 21, the percentage of cells expressing GFP has not decreased, indicating there is no silencing of the reporter gene.
GFP ioGlutamatergic Neurons form structural neuronal networks by day 11
GFP ioGlutamatergic Neurons mature rapidly, show glutamatergic neuron morphology and form structural neuronal networks over 11 days, comparable to the wild-type control. Day 1 to 11 post thaw; 10X magnification.
GFP ioGlutamatergic Neurons demonstrate gene expression of neuronal-specific and glutamatergic-specific markers following deterministic programming
Gene expression analysis demonstrates that GFP ioGlutamatergic Neurons (GFP) and wild-type ioGlutamatergic Neurons (WT) lack the expression of pluripotency marker OCT4 (POU5F1) at day 11, while robustly expressing pan-neuronal (TUBB3 and SYP) and glutamatergic-specific (VGLUT1 and VGLUT2) markers, as well as the glutamate receptor GRIA4.
Gene expression levels were assessed by RT-qPCR, data normalised to HMBS; cDNA samples of the parental human iPSC line (iPSC) were included as reference. Data represents day 11 post-revival samples.
Easy-to-use co-culture protocol for GFP ioGlutamatergic Neurons with ioMicroglia
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.







Hoescht(blue)_day12v2.png?width=604&name=bit.bio_ioGlutamatergic%20Neurons_20xMAP2(red)Hoescht(blue)_day12v2.png)
Hoescht(blue)TUBB3(blue)_day4.jpg?width=604&name=bit.bio_ioGlutamatergic%20Neurons_60xMAP2(red)Hoescht(blue)TUBB3(blue)_day4.jpg)