







cat no | io6023
ioSkeletal Myocytes DMD Exon 51 Deletion
Human iPSC-derived Duchenne muscular dystrophy model
-
Cryopreserved human iPSC-derived cells powered by opti-ox that are ready for experiments in days
-
Study Duchenne muscular dystrophy in a human in vitro model engineered with a DMD exon 51 deletion
-
Consistent, functional model with genetically matched wild type control, suitable for experiments in 2D and 3D muscle bundles

Human iPSC-derived Duchenne muscular dystrophy model

ioSkeletal Myocytes DMD Exon 51 Deletion disease model cells express skeletal muscle cell specific markers and lack expression of dystrophin, demonstrating a Duchenne muscular dystrophy phenotype
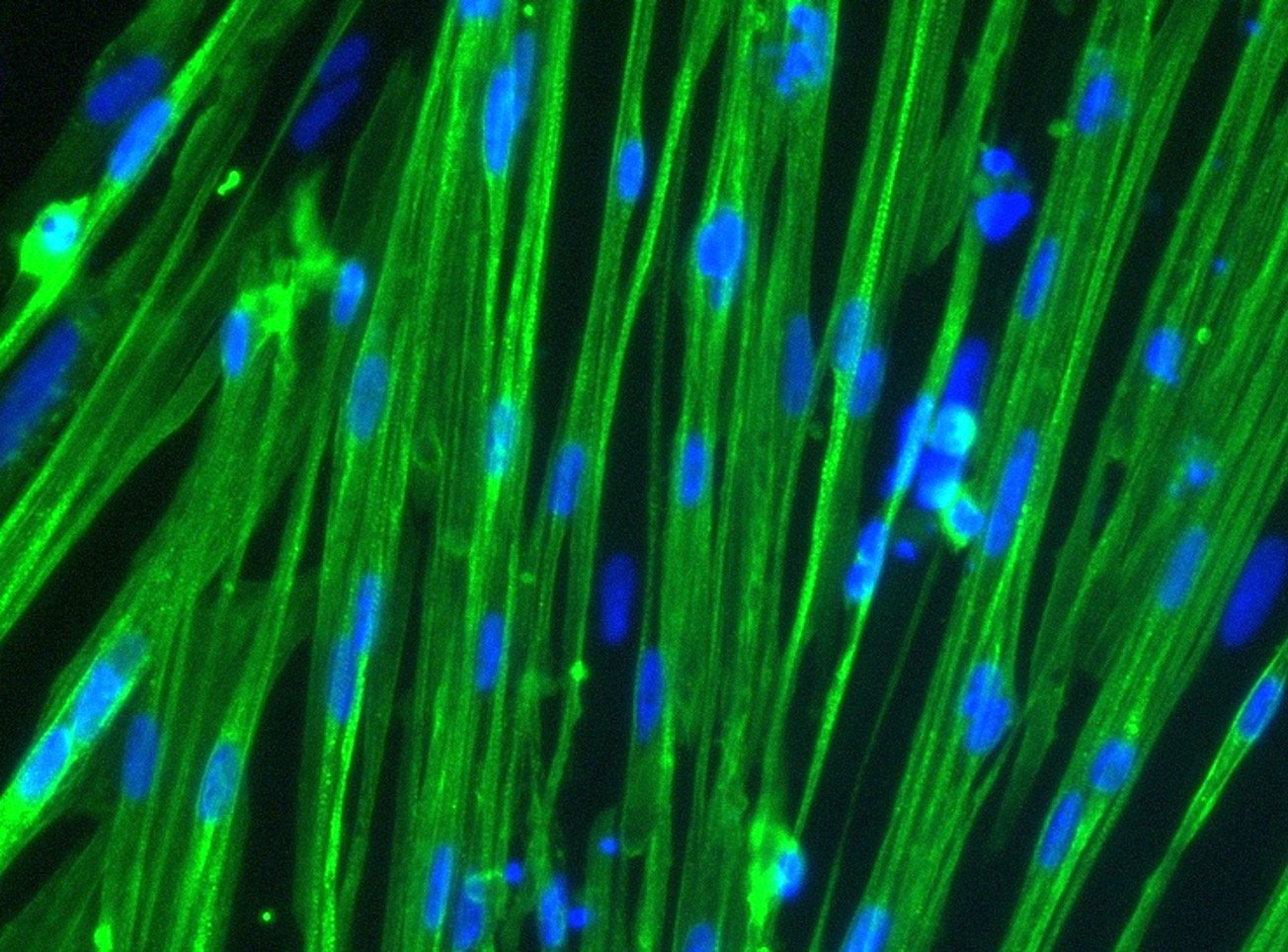
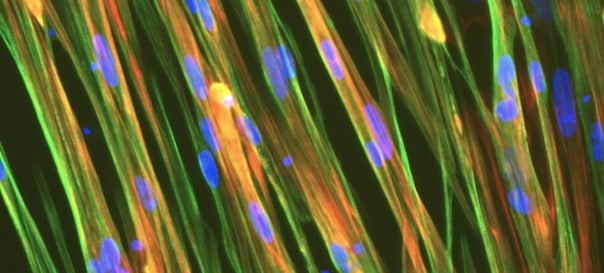
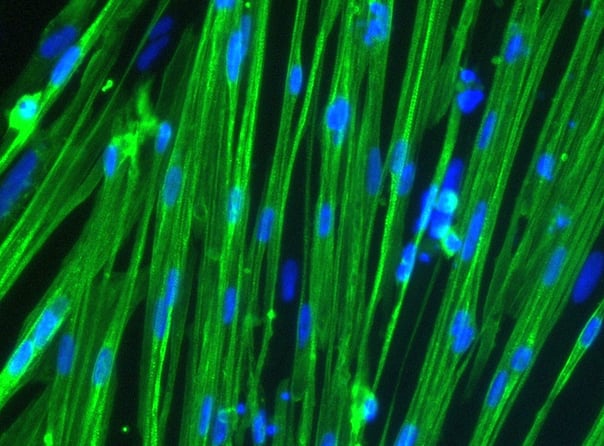
Immunocytochemistry staining at day 10 post revival demonstrates robust expression of desmin, a component of the contractile apparatus, and no expression of dystrophin in the ioSkeletal Myocytes DMD Del Ex51/Y disease model cells, whereas ioSkeletal Myocytes, the wild type isogenic control, express both markers (upper panel). Robust expression of myosin heavy chain (MHC) and the muscle transcription factor myogenin is observed in both ioSkeletal Myocytes DMD Del Ex51/Y and ioSkeletal Myocytes (lower panel). Anti-dystrophin antibody clone 2C6 (MANDYS106).

ioSkeletal Myocytes DMD Exon 51 Deletion disease model cells demonstrate classical skeletal myocytes morphology
ioSkeletal Myocytes DMD Exon 51 Deletion form elongated, multinucleated myocytes over 10 days, comparable to the wild-type ioSkeletal Myocytes isogenic control. Day 3 to 10 post-revival; 10X, scale bar 400 um.

ioSkeletal Myocytes DMD Exon 51 Deletion disease model cells demonstrate gene expression of key myogenic markers following deterministic cell programming
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.

-1%20(1).png?width=604&name=horizontal_ioSkeletal-Myocytes-Muscle-Bundle-Biond-ICC-SAA-Actin%20(1)-1%20(1).png)
.png?width=604&name=ISSCR24-DMD-Exon44-3D-muscle-bundle%20(1).png)





/ioSkeletal-Myocytes-DMD-Exon-52-Deletion-hero.webp?width=1276&height=1190&name=ioSkeletal-Myocytes-DMD-Exon-52-Deletion-hero.webp)



