






cat no | io1041
ioMotor Neurons SOD1 G93A/G93A
Human iPSC-derived ALS disease model
- Cryopreserved human iPSC-derived cells powered by opti-ox, that are ready for experiments in days
- Engineered to enable investigations into the impact of mutant SOD-1 protein on neurodegenerative disease
- Clump free, highly-pure motor neurons, that form functional neuronal networks in co-culture with astrocytes

Human iPSC-derived ALS disease model

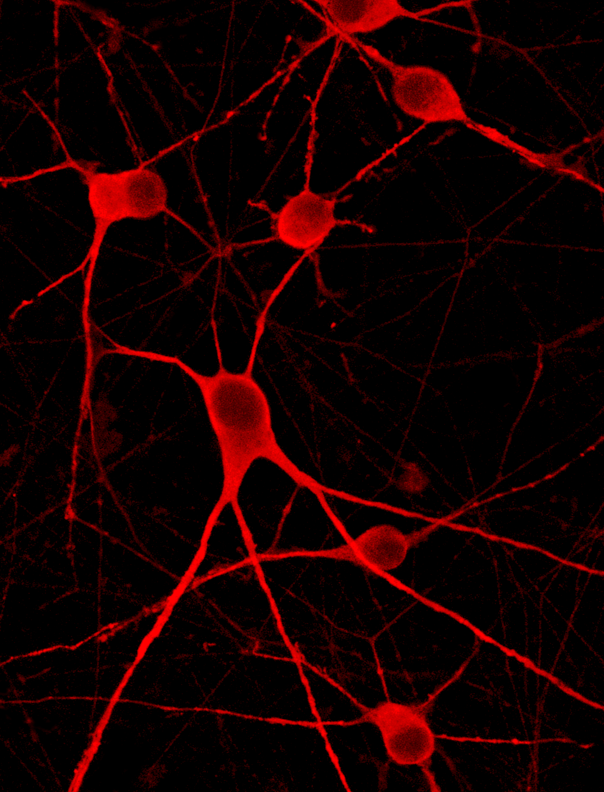
ioMotor Neurons SOD1 G93A/G93A form a homogenous neuronal network by day 4
ioMotor Neurons SOD1 G93A/G93A rapidly acquire a motor neuronal phenotype, forming homogenous neuronal networks, without clumping of cells. Compared to the genetically matched wild type control, ioMotor Neurons. Day 1 to 11 post thawing; 100X magnification.

ioMotor Neurons SOD1 G93A/G93A express motor neuron-specific markers with protein expression highly reminiscent to the genetically matched control
Immunofluorescent staining on post-revival day 11 demonstrates similar homogenous expression of pan-neuronal protein TUBB3, motor neuron specific marker ISL2 and the cholinergic marker VAChT in ioMotor Neurons SOD1 G93A/G93A compared to the genetically matched control, ioMotor Neurons.

ioMotor Neurons SOD1 G93A/G93A express motor neuron-specific markers with protein expression highly reminiscent to the genetically matched control
Immunofluorescent staining on post-revival day 11 demonstrates similar homogenous expression of pan-neuronal protein MAP2, motor neuron specific marker HB9 and the cholinergic marker VAChT in ioMotor Neurons SOD1 G93A/G93A compared to the genetically matched control, ioMotor Neurons.

ioMotor Neurons SOD1 G93A/G93A demonstrate gene expression of neuronal-specific and motor neuron-specific markers following deterministic programming
Gene expression analysis demonstrates that ioMotor Neurons SOD1 G93A/G93A and the genetically matched control (WT) lack the expression of pluripotency makers (NANOG and OCT4), at day 11, whilst robustly expressing pan-neuronal (MAP2), cholinergic (CHAT and VACHT) and motor neuron-specific (MNX1 and ISL2) markers. Gene expression levels were assessed by RT-qPCR (data expressed relative to the parental hiPSC control (iPSC Control), normalised to HMBS). Data represents day 11 post-revival samples.

Disease-related SOD1 is expressed in ioMotor Neurons SOD1 G93A/G93A following deterministic programming
Gene expression analysis demonstrates that ioMotor Neurons SOD1 G93A/G93A and the genetically matched control (WT) express the SOD1 gene encoding the Superoxide dismutase 1 protein. Gene expression levels were assessed by RT-qPCR (data expressed relative to the parental hiPSC control (iPSC Control), normalised to HMBS). Data represents day 11 post-revival samples.

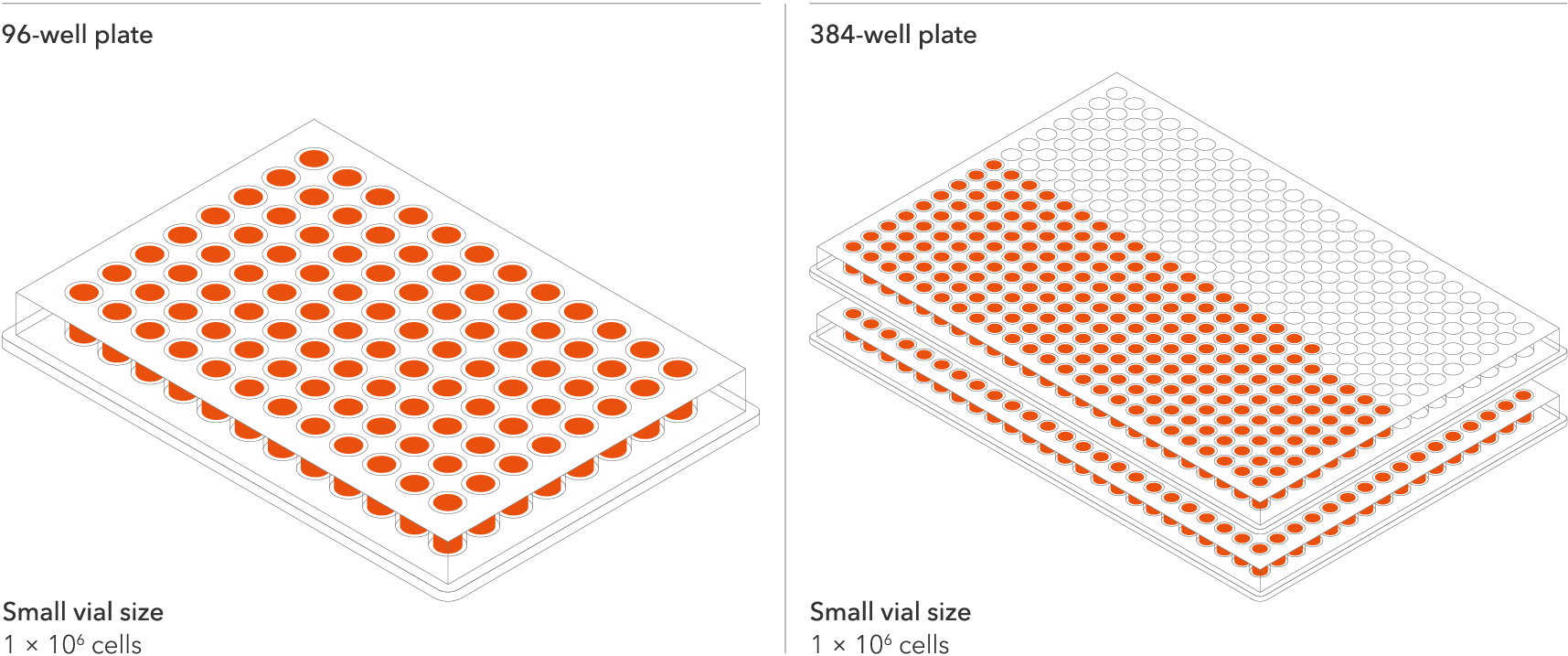
Do more with every vial
The seeding density of our human iPSC-derived ioMotor Neurons and related disease models has been optimised and validated to a recommended seeding density of 30,000 cells/cm². This means scientists can do more with every vial and expand experimental design within budget without losing out on quality. Resulting in more experimental conditions, more repeats, and more confidence in the data. One Small vial can plate a minimum of 0.7 x 24-well plate, 1 x 96-well plate, or 1.5 x 384-well plate.
Vial limit exceeded
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.