20.02.2023 | Published by bit.bio
.png)
Human iPSC-derived excitatory neurons provide helpful physiologically relevant cell models to investigate neurodevelopmental and neurodegenerative disorders, although achieving healthy neuronal cultures can be challenging. bit.bio central nervous system (CNS) scientist Dr Kaiser Karim has studied the genomic mechanisms governing reprogramming of human iPSCs into glutamatergic neurons and contributed to research investigating the effects of energetic substrate availability on global neural network behaviour.
In this blog, Kaiser shares his top tips on glutamatergic cell culture to help you overcome some of the most common issues. By using careful handling to prevent cell detachment, promoting cell adherence, perfecting routine media changes, and using the right cell density, it’s possible to achieve regular and consistent cell culture success!
Go straight to the top tips!
- Top tip #1: Careful handling can prevent cell detachment from culture surfaces
- Top tip #2: Use PDL in borate buffer-Geltrex to enhance cell adherence
- Top tip #3: Closely monitor media change timings to ensure successful cultures
- Top tip #4: Use the right cell density to get better-quality data from your experiments
What are glutamatergic neurons?
Glutamatergic neurons are cells that produce the common excitatory neurotransmitter glutamate, ‘exciting’ other neurons. These cells can be found throughout the CNS, for example within the cortex, where they are abundant, as well as the spinal cord and hypothalamus. Glutamatergic neurons contribute to a number of CNS-related functions such as cognition, memory, learning, and sensory perception1. They have also been implicated in a number of neurodegenerative diseases, including Alzheimer’s disease2.
The human iPSC-derived ioGlutamatergic Neurons provided by bit.bio are reprogrammed iPSC-derived cells and provide a highly relevant and consistent in vitro human model3. They form networks of postsynaptic dendrites and presynaptic axon projections, recapitulating the type of human neuron-to-neuron communication networks found in the body4.
"With such complex and fragile structures, culturing glutamatergic neurons in vitro requires advanced planning and special attention", cautions Kaiser, and so here are some top tips for success.
Top tip #1: Careful handling can prevent cell detachment from culture surfaces
Cell detachment is a common problem scientists face when culturing glutamatergic neurons. ioGlutamatergic Neurons, for example, can form neurites after only two days in culture and are ready for experiments in as little as 11 days, although they can be fragile. “As neuronal cell bodies form connections across the culture plate via neurites, the disruption of one neuron can impact the entire culture,” says Kaiser. With careful handling, however, the development of these neuronal networks will increase and mature over time.
There are 4 key considerations surrounding medium changes that can reduce the risk of cell detachment:
- Avoid aspirating too close to neurons, as this can potentially create a ‘tear’ in neuronal cultures. Kaiser comments: “Unfortunately, once there is a tear and disruption to the culture, the glutamatergic neurons will suffer permanent damage and eventually peel off.” The early stages of glutamatergic neuron culture can be especially vulnerable, as 90% medium changes are required. Cells can be disrupted if the media is changed too quickly or aspiration occurs too close to the neurons.
- Every time you add medium to your glutamatergic neuron cultures, target a different location within the plate or well, and keep track of where it’s been added. For example, on Day 0, you could add medium to the left side of the culture well, Day 2 on the right side, Day 4 at the top, etc. The cells’ neuronal networks are more likely to detach if they are continuously fed in the same place. This is because the pressure of the liquid going into the plates/wells can weaken cell attachment as well as cells’ extracellular matrix (ECM).
- Another consideration is to tilt the plate to aspirate spent medium from the culture wells, when carrying out a 90% medium change, this helps to ensure that the pipette tip doesn’t touch the culture.
- Finally, if using a stripette, set it at a very low flow rate to prevent disruption of the neuronal network, gently handling both the cell culture plate and liquid flow with careful attention at all times.
 Careful handling and media changes that avoid causing a ‘tear’ in the culture can prevent cells from lifting off surfaces and improve glutamatergic neuronal cell culture success. Tilting the cell culture plate, as shown here, can help prevent direct damage to the cells caused by adding fresh medium to wells.
Careful handling and media changes that avoid causing a ‘tear’ in the culture can prevent cells from lifting off surfaces and improve glutamatergic neuronal cell culture success. Tilting the cell culture plate, as shown here, can help prevent direct damage to the cells caused by adding fresh medium to wells.
Top tip #2: Use PDL in borate buffer-Geltrex to enhance cell adherence
Human iPSC-derived cell cultures generally benefit from ECM coatings on the cell culture plate surface. After much testing and optimisation, we’ve found that poly-d-lysine (PDL)-Geltrex works very successfully for iPSC-derived human neurons. PDL is a synthetic, non-ECM coating molecule that improves cell attachment to surfaces via electrostatic interactions. We resuspend PDL in borate buffer rather than water, which in our experience is a critical factor in achieving success. Geltrex is a soluble basement extracellular matrix-derived coating that contains a cocktail of laminin, collagen IV, heparin sulphate proteoglycans, and entactin/nidogen. The presence of such molecules plays a role in recapitulating the physiological microenvironment, promoting cell-cell interactions and network activity5,6. With this double layer surface coating, ioGlutamatergic Neurons can be cultured for over 2-3 weeks, to enable functional studies into neuronal maturation and activity, explains Kaiser.
Top tip #3: Closely monitor media change timings to ensure successful cultures
Getting your media change protocol right throughout the entire length of a cell culture is essential, including timings. For ioGlutamatergic Neurons, Kaiser thaws the cells from cryopreservation and seeds them onto cell culture surfaces on a Monday. The cells then undergo a stabilisation phase in the presence of doxycycline (dox) for four days. During this time, 90% medium changes are necessary every 48 hours. So, for example, he carries out a 90% medium change on a Wednesday (i.e., day two post-thawing, with dox and DAPT), followed by the last 90% medium change on a Friday (day 4, without dox). Once the stabilisation phase is over, from day 6/7 onwards, Kaiser recommends replacing 50% of the medium every 48 hours (see diagram below for further steps).
“This protocol is also helpful for those times when ‘life happens’, and you can’t make it into the lab at the weekend to do a media change,” Kaiser adds.
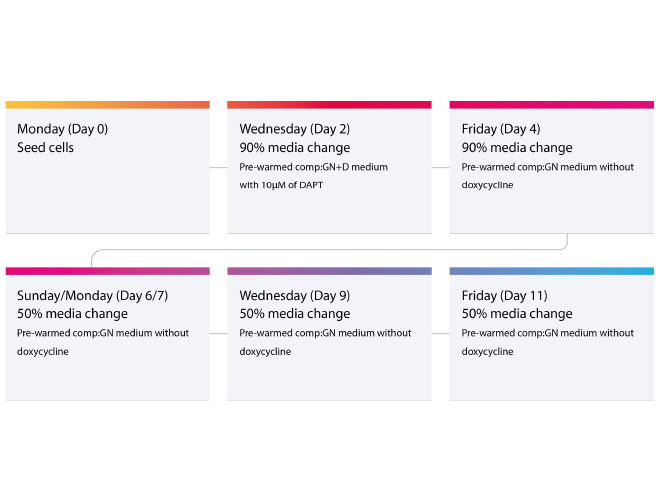 Suggested media change cycle for bit.bio’s iPSC-derived glutamatergic neurons. This workflow can be repeated for the length of cell culture time. Further details can also be found in the bit.bio ioGlutamatergic Neurons’ User Manual.
Suggested media change cycle for bit.bio’s iPSC-derived glutamatergic neurons. This workflow can be repeated for the length of cell culture time. Further details can also be found in the bit.bio ioGlutamatergic Neurons’ User Manual.
Top tip #4: Use the right cell density to get better-quality data from your experiments
Using the right cell seeding density of cryopreserved glutamatergic neurons generally makes it easier to isolate, stain, image and analyse individual neurons, dendrites, axons and synapses, says Kaiser. However, commonly recommended seeding densities of >100,000/cm2 often make it challenging to carry out these methods.
bit.bio’s ioGlutamatergic Neurons can be seeded out at a much lower density - a minimum of 30,000 cells/cm2, and are compatible with plates ranging from 6- to 384-well formats. “We are confident in the quality of our neurons and recommend cell seeding at 30,000 cells/cm2 for everyday experiments,” says Kaiser.
At this density, ioGlutamatergic Neurons can be used in routine experiments and have been pre-validated for immunocytochemistry, qPCR, and RNAseq. The cells have also been validated for morphology characterisation, including brightfield imaging and quantitative live-cell imaging and analysis (like neurite outgrowth). Furthermore, at this density, the cells are cost-effective without impacting quality.
“For complex experiments, such as analysing neuronal network activity using neuronal electrophysiology or calcium signalling, higher seeding densities may be necessary, including additional optimisation,” adds Kaiser.
Summary
Applying the tips above to prevent cell detachment and enhance cell adherence, combined with well-regulated and timed media changes and the right cell density, should help make your glutamatergic neuron culture successful from the start. For more tips and information on ioGlutamatergic Neurons cell culture, check out this helpful step-by-step video.
Do you have any cell culture tips to share? We’d love to hear from you!
About Kaiser Karim, PhD
Kaiser completed his PhD in Clinical Neurosciences at the University of Cambridge, under the supervision of Dr Mark Kotter (NIHR Clinician Scientist, Consultant in Neurosurgery, University of Cambridge and CEO bit.bio), where he studied the genomic mechanisms that govern reprogramming of human iPSCs into induced glutamatergic neurons. This work followed the pivotal 2017 paper published by Mark Kotter’s academic research group. He has also contributed to work looking into the effects of energetic substrate availability on global neural network behaviour. Kaiser’s research has provided a deeper understanding of bit.bio’s ioGlutamatergic Neurons and helped lay the foundations for its commercialisation.
 Kaiser KarimScientist at bit.bio
Kaiser KarimScientist at bit.bio
References
- Sanacora G, Zarate C, Krystal J et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7, 426–437 (2008). https://doi.org/10.1038/nrd2462.
- Gasiorowska A, Wydrych M, Drapich P, et al. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front Aging Neurosci. 2021 Jul 13;13:654931. doi: https://doi.org/10.3389/fnagi.2021.654931.
- Pawlowski M, Ortmann D, Bertero A, et al. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Reports. 2017 8(4):803-812. https://doi.org/10.1016/j.stemcr.2017.02.016.
- Tourigny DS, Abdul Karim MK, Echeveste R, & Kotter, RN. Energetic substrate availability regulates synchronous activity in an excitatory neural network. PLOS ONE. 2019 14(8), e0220937. https://doi.org/10.1371/journal.pone.0220937.
- Barros CS, Franco SJ, Müller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 2011 1;3(1):a005108. https://doi.org/10.1101/cshperspect.a005108.
- Lam D, Enright HA, Cadena J et al. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci Rep. 2019 9: 4159. https://www.nature.com/articles/s41598-019-40128-1.