06.06.2025 | Published by bit.bio
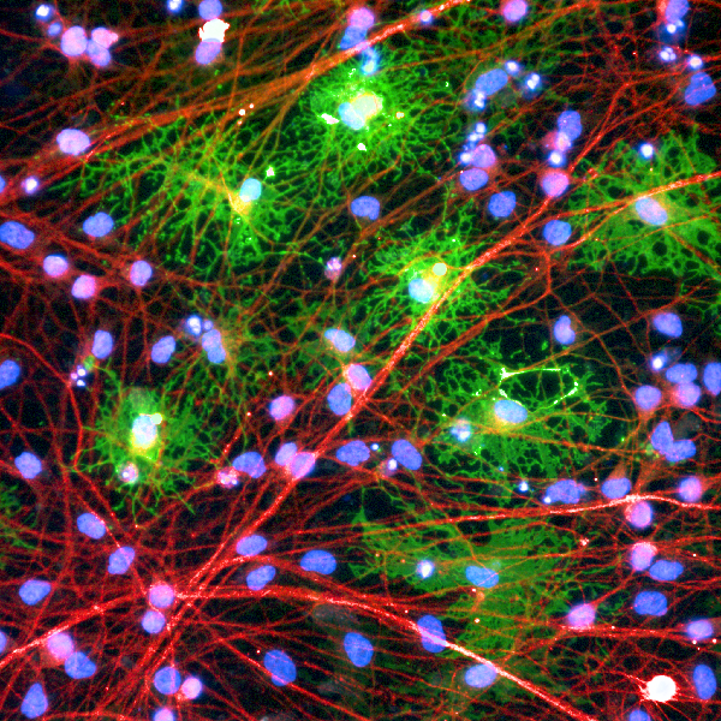
ioOligodendrocyte-like cells are ideal for the quick and scalable screening of compounds that modulate myelination. After more than a decade of studying modern drug development practices, industry analyst Jack Scannell mused that “successful drug discovery is like finding oases of safety and efficacy in chemical and biological deserts.”1
For the greater part of a century, the pharmaceutical industry has struggled with extremely low success rates in bringing new candidate therapeutics to market. Technological and scientific breakthroughs have greatly advanced our understanding of human health and disease, yet the cost of therapeutic development continues to rise while the flow of new and successful drugs through development pipelines remains a trickle.
The slow pace of pharmaceutical progress is particularly evident when considering therapeutics to treat neurodegenerative diseases. Almost all drugs entering clinical trials for neurodegenerative disease fail to reach the market.4 As a result, there is a lack of curative treatments for progressively debilitating neurological conditions such as Multiple Sclerosis (MS) and leukodystrophies. Fortunately, as Scannell points out, small changes in preclinical drug development practices can have large effects on drug development success and productivity.1-5
One such change is to improve the translational value of drug screening models.1-5 Traditionally, conditions like MS have been studied using a combination of animal models and patient samples.6 However, significant inter-species differences erode the validity of animal models, and patient-derived tissue is both hard to come by and is prone to substantial variation among samples. Therefore drug development in this space has been greatly limited by the poor scalability and translational validity of current models.
Human induced pluripotent stem cells (iPSC) have significant potential to improve drug development productivity by providing researchers with disease-relevant, human cells at the scale needed for high throughput drug screening. For researchers studying demyelinating diseases like MS, human iPSC-derived oligodendrocyte-like cells can be transformative.
What are ioOligodendrocyte-like cells?
ioOligodendrocyte-like cells are highly characterised cells that resemble a pre-myelinating oligodendrocyte state, that are deterministically programmed from human iPSC using opti-ox™ technology, which allows for the faithful execution of cell fate-defining genetic programs. These cells bear phenotypic resemblance to cells from the oligodendroglial lineage, a group of cells that are critically involved in neuronal health and function. Specifically, the oligodendroglial lineage gives rise to astrocytes, Schwann cells, and oligodendrocytes, the latter of which is located in the central nervous system and is responsible for ensheathing neuronal axons in a highly conductive material known as myelin.7 In doing so, oligodendrocytes enable fast propagation of action potentials and maintenance of axonal integrity.7
 Figure 1: Oligodendroglial Lineage Markers. This simplified diagram shows key gene expression profiles of oligodendroglial cells as they differentiate from an early multipotent stage (perinatal progenitor) into mature myelinating oligodendrocytes.7
Figure 1: Oligodendroglial Lineage Markers. This simplified diagram shows key gene expression profiles of oligodendroglial cells as they differentiate from an early multipotent stage (perinatal progenitor) into mature myelinating oligodendrocytes.7
Notably, the failure of oligodendrocytes to myelinate neurons is characteristic of demyelinating diseases like MS.6 Therefore, the oligodendroglial lineage has gained considerable attention in recent years as a potential therapeutic target, with researchers hoping to stimulate myelination as a means to slow or prevent disease progression.
ioOligodendrocyte-like cells exhibit a unique gene expression profile that resembles a pre-myelinating oligodendrocyte. Critically, these cells demonstrate expression of key markers that are typically associated with pre-myelinating and myelinating oligodendrocytes (Fig. 1), such as myelin basic protein (MBP), proteolipid protein 1 (PLP1), 2',3'-Cyclic nucleotide 3'-phosphodiesterase (CNP), and others.
ioOligodendrocyte-like cells are ideal for the quick and scalable screening of compounds that modulate myelination.
Contact our team to test a vial of ioOligodendrocyte-like cells today
ioOligodendrocyte-like cells solve key challenges in preclinical drug development
Drug development frequently involves large-scale screening of prospective compounds using reductionist in vitro models. While such models lack much of the complexity that living organisms possess, this approach allows researchers to quickly and efficiently hone in on compounds that elicit the desired phenotype. Follow-up studies in more complex model systems can then be done using only the most promising of candidates, saving researchers time and resources.
As stated above, researchers studying demyelinating diseases have lacked a human-relevant system that could be used for this type of large-scale drug screening.6 Not only are primary cells difficult to source, but they are typically not amenable to expansion and can vary greatly in mutational background between donors. In recent years, many researchers have turned to iPSCs as a potentially scalable source of human oligodendrocytes.
According to Marcos Herrera Vaquero, Ph.D., Senior Scientist at bit.bio, “deriving human oligodendroglial cells from iPSCs is no small task. Most current protocols rely on one of two processes: either forward reprogramming using lentiviral-delivered transcription factors, or the process of directed differentiation which attempts to mimic developmental processes.” (Table 1).8
While effective, lentiviral delivery has significant drawbacks that include the random integration of genetic components into the target cell’s genome. “This approach can potentially cause disruption of native genes, silencing of the inserted gene, and overall stochastic effects on gene expression that vary from cell-to-cell,” explains Herrera Vaquero. Such variation can greatly erode experimental reproducibility, impacting assay quality.
Table 1: Published protocols for generating iPSC-derived oligodendrocytes by directed differentiation and forward reprogramming lentiviral-delivered transcription factors
|
Protocol |
Starting Material |
Approach |
Timecourse |
% MBP expressing cells |
Reference |
|
Douvaras and Fossati, 2015 |
iPSCs |
Directed Differentiation |
95 days |
Up to 35% |
|
|
Ehrlich et al, 2017 |
Neuronal Progenitor Cells (NPCs) |
Lentiviral transduction of SOX10, OLIG2 and NKX6.2 |
35 days |
Up to 30% |
|
|
Garcia-Leon et al, 2018 |
iPSC-derived NPCs |
Lentiviral transduction of SOX10 |
12 days |
Up to 21% |
|
|
Garcia-Leon et al, 2020 |
Pluripotent Stem Cells |
Lentiviral (10 days) or gene-edited (3 months) |
22 days |
Up to 20% |
Similarly, directed differentiation is a naturally stochastic approach to cell differentiation that requires a high degree of technical training, can be resource and time-intensive, and is often difficult to reproduce across time and institutions. “These protocols require a considerable time commitment that may span months, and that’s if everything goes well and is done perfectly by the book,” adds Herrera Vaquero. One commonly used protocol, for example, requires 95 days to produce cells expressing the MBP oligodendrocyte marker typical of pre-myelinating and myelinating oligodendrocytes.9
Cumulatively, the difficulties associated with sourcing oligodendroglial lineage cells through lentiviral or directed differentiation methods can reduce experimental reproducibility and limit scalability in drug development applications.14
On the other hand, as opti-ox provides precise control over cell-fate defining transcription factors, and the expression of these transcription factors is switched on before cryopreservation, ioOligodendrocyte-like cells are ready for experimentation just one day after thawing. Cells on day 1, according to their morphology resemble oligodendrocyte progenitor cells (OPCs; multipotent cells that pervade the central nervous system and are recruited to sites of damage, where they differentiate into myelinating oligodendrocytes).
 Figure 2: Morphology of ioOligodendrocyte-like cells. Upon deterministic programming, cells show rapid morphological changes, acquiring an oligodendrocyte progenitor cell-like (OPC-like) morphology by day 1 post-revival. This morphology is typified by a bipolar shape. By day 8, cells have matured and display an oligodendrocyte-like morphology, characterised by multiple branched processes. Brightfield images show day 1 and day 8 post-thawing; scale bar: 400 μm.
Figure 2: Morphology of ioOligodendrocyte-like cells. Upon deterministic programming, cells show rapid morphological changes, acquiring an oligodendrocyte progenitor cell-like (OPC-like) morphology by day 1 post-revival. This morphology is typified by a bipolar shape. By day 8, cells have matured and display an oligodendrocyte-like morphology, characterised by multiple branched processes. Brightfield images show day 1 and day 8 post-thawing; scale bar: 400 μm.
By day 8, ioOligodendrocyte-like cells exhibit expression of MBP and other key markers. The speedy acquisition of this cell identity means that researchers can quickly assess how compounds affect the expression of key myelin-associated proteins, like MBP.
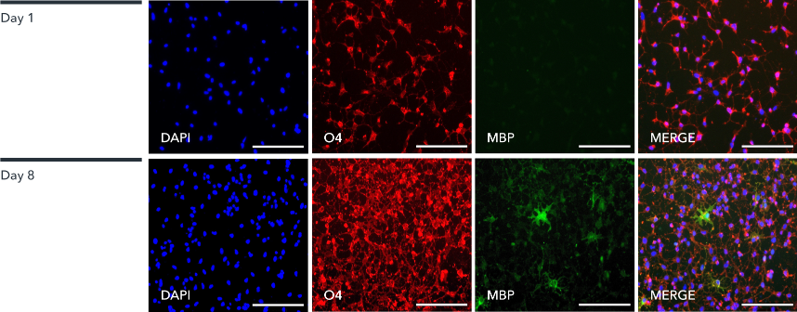
Figure 3: Marker expression in ioOligodendrocyte-like cells. Immunofluorescent staining of the cells at day 1 (upper panel) and day 8 (lower panel) post-revival. At day 1, the cells are positive for the oligodendrocyte-specific marker O4 (red), DAPI counterstain also shown (blue). At day 8, ioOligodendrocyte-like cells show an increased complexity and are positive for O4 (red), myelin basic protein (MBP) (green), DAPI counterstain also shown (blue). 100X magnification; scale bar: 100 μm.
And, like all ioCells, ioOligodendrocyte-like cells are highly defined and consistent from lot-to-lot, enabling researchers to confidently scale up to drug screens involving many millions of cells without worrying about statistical noise from inter-lot variability.
Finding a source of cells with the right gene expression profile is essential to the development of physiologically relevant assays. Contact our team to request gene expression data for ioOligodendrocyte-like cells.
Pushing demyelinating disease research forward
By providing researchers with a quick, reliable, and scalable source of human cells that express key oligodendrocyte markers within days, they are enabled to build efficient large-scale compound screens for demyelinating disease. With their potential to greatly improve modern drug development, we hope that ioOligodendrocyte-like cells will be an essential tool powering new advancements in our ability to help patients suffering from demyelinating disease.
Learn more about ioOligodendrocyte-like cells
References
- Scannell, Jack W., et al. “Predictive Validity in Drug Discovery: What It Is, Why It Matters and How to Improve It.” Nature Reviews Drug Discovery, 4 Oct. 2022, https://doi.org/10.1038/s41573-022-00552-x.
- Ringel, Michael S., et al. “Breaking Eroom’s Law.” Nature Reviews Drug Discovery, vol. 19, no. 12, 16 Apr. 2020, pp. 833–834, https://doi.org/10.1038/d41573-020-00059-3.
- Scannell, Jack W., et al. “Diagnosing the Decline in Pharmaceutical R&D Efficiency.” Nature Reviews Drug Discovery, vol. 11, no. 3, Mar. 2012, pp. 191–200, https://doi.org/10.1038/nrd3681.
- Gribkoff, Valentin K., and Leonard K. Kaczmarek. “The Need for New Approaches in CNS Drug Discovery: Why Drugs Have Failed, and What Can Be Done to Improve Outcomes.” Neuropharmacology, vol. 120, 1 July 2017, pp. 11–19, https://doi.org/10.1016/j.neuropharm.2016.03.021.
- Scannell, Jack W., and Jim Bosley. “When Quality Beats Quantity: Decision Theory, Drug Discovery, and the Reproducibility Crisis.” PLOS ONE, vol. 11, no. 2, 10 Feb. 2016, p. e0147215, https://doi.org/10.1371/journal.pone.0147215.
- Balestri, Sonia, et al. “The Current Challenges for Drug Discovery in CNS Remyelination.” International Journal of Molecular Sciences, vol. 22, no. 6, 12 Mar. 2021, p. 2891, https://doi.org/10.3390/ijms22062891.
- Amaral, Ana I, et al. “Oligodendrocytes: Development, Physiology and Glucose Metabolism.” Advances in Neurobiology, 1 Jan. 2016, pp. 275–294, https://doi.org/10.1007/978-3-319-45096-4_10.
- Goldman, S. A., and N. J. Kuypers. “How to Make an Oligodendrocyte.” Development, vol. 142, no. 23, 1 Dec. 2015, pp. 3983–3995, https://doi.org/10.1242/dev.126409.
- Douvaras, Panagiotis, and Valentina Fossati. “Generation and Isolation of Oligodendrocyte Progenitor Cells from Human Pluripotent Stem Cells.” Nature Protocols, vol. 10, no. 8, 2 July 2015, pp. 1143–1154, https://doi.org/10.1038/nprot.2015.075.
- Ehrlich, Marc, et al. “Rapid and Efficient Generation of Oligodendrocytes from Human Induced Pluripotent Stem Cells Using Transcription Factors.” Proceedings of the National Academy of Sciences, vol. 114, no. 11, 28 Feb. 2017, pp. E2243–E2252, https://doi.org/10.1073/pnas.1614412114.
- Pawlowski, Matthias, et al. “Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes.” Stem Cell Reports, vol. 8, no. 4, 23 Mar. 2017, pp. 803–812, https://doi.org/10.1016/j.stemcr.2017.02.016.
- Juan Antonio García-León, et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Vol. 10, no. 2, 13 Feb. 2018, pp. 655–672, https://doi.org/10.1016/j.stemcr.2017.12.014.
- García-León, Juan Antonio, et al. “Generation of Oligodendrocytes and Establishment of an All-Human Myelinating Platform from Human Pluripotent Stem Cells.” Nature Protocols, vol. 15, no. 11, 1 Nov. 2020, pp. 3716–3744, https://doi.org/10.1038/s41596-020-0395-4.
- Volpato, Viola, et al. “Reproducibility of Molecular Phenotypes after Long-Term Differentiation to Human IPSC-Derived Neurons: A Multi-Site Omics Study.” Stem Cell Reports, vol. 11, no. 4, 9 Oct. 2018, pp. 897–911, pubmed.ncbi.nlm.nih.gov/30245212/, https://doi.org/10.1016/j.stemcr.2018.08.013.