Publication
CRISPR-Cas9 knockout screen in iPSC-derived Neurons identifies new Alzheimer’s disease druggable target
Human iPSC-derived neurons powered by opti-ox, constitutively expressing Cas9, enable a pooled CRISPR screen targeting the human druggable genome. KAT2B is identified as a target from the screen, which when inhibited in the presence of Tunicamycin attenuates neuronal cell death.
Read more
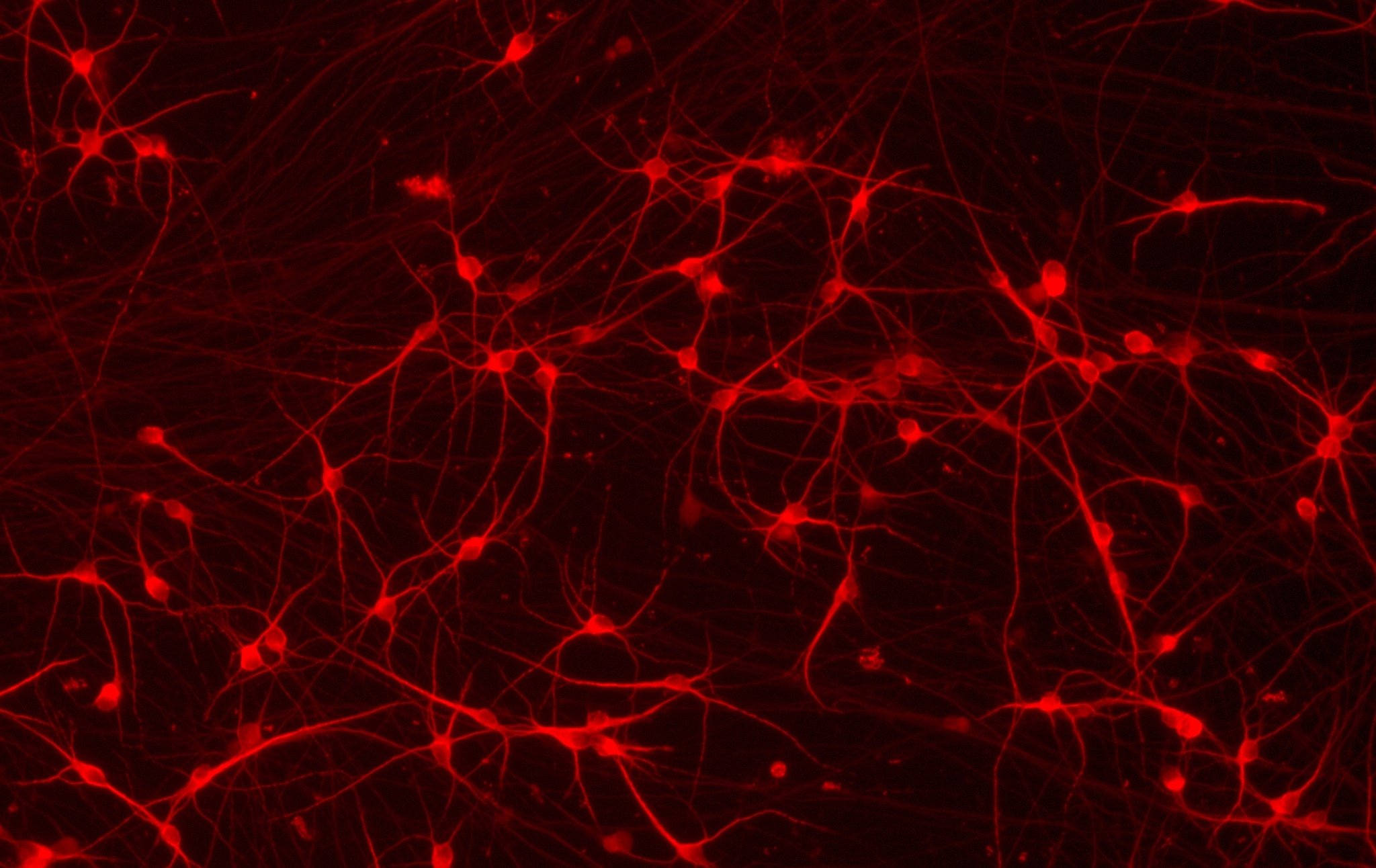
Human iPSC-derived neurons powered by opti-ox, constitutively expressing Cas9, enable a pooled CRISPR screen targeting the human druggable genome. KAT2B is identified as a target from the screen, which when inhibited in the presence of Tunicamycin attenuates neuronal cell death.
This study aimed to find therapeutic targets for the treatment of neurodegenerative conditions, specifically looking to identify genes critical for the unfolded protein response (UPR). The UPR causes cell death in the presence of excessive unfolded or misfolded proteins, common in neurodegenerative diseases like Alzheimer’s disease.
To find these proteins, the authors performed a CRISPR knockout screen in an opti-ox powered iPSC-derived neuron with stable expression of Cas9 that targeted 4401 genes in the human genome that are currently druggable. UPR was induced in the screen by Tunicamycin treatment. The results identified 188 genes that appeared to be involved in UPR-mediated cell death. A focused arrayed screen targeting the 38 most promising hits refined the results to 13 genes with high potential for UPR association. A series of follow-up assays identified KAT2B, which when inhibited by treatment with L-Moses, attenuated UPR-mediated neuronal cell death.
The authors conclude that their use of opti-ox powered iPSC-derived neurons engineered to express the Cas9 protein solved the challenges of Cas9 delivery to neuronal cells, excessive silencing of Cas9, and the heterogeneity of other iPSC-derived neuronal cells. The authors also speculate that solving these challenges also solves the root cause behind the low number of current literature featuring CRISPR screens in human neurons.
Read more
To find these proteins, the authors performed a CRISPR knockout screen in an opti-ox powered iPSC-derived neuron with stable expression of Cas9 that targeted 4401 genes in the human genome that are currently druggable. UPR was induced in the screen by Tunicamycin treatment. The results identified 188 genes that appeared to be involved in UPR-mediated cell death. A focused arrayed screen targeting the 38 most promising hits refined the results to 13 genes with high potential for UPR association. A series of follow-up assays identified KAT2B, which when inhibited by treatment with L-Moses, attenuated UPR-mediated neuronal cell death.
The authors conclude that their use of opti-ox powered iPSC-derived neurons engineered to express the Cas9 protein solved the challenges of Cas9 delivery to neuronal cells, excessive silencing of Cas9, and the heterogeneity of other iPSC-derived neuronal cells. The authors also speculate that solving these challenges also solves the root cause behind the low number of current literature featuring CRISPR screens in human neurons.
Pavlou et al.
Nature Scientific Reports
2023