cat no | io1014
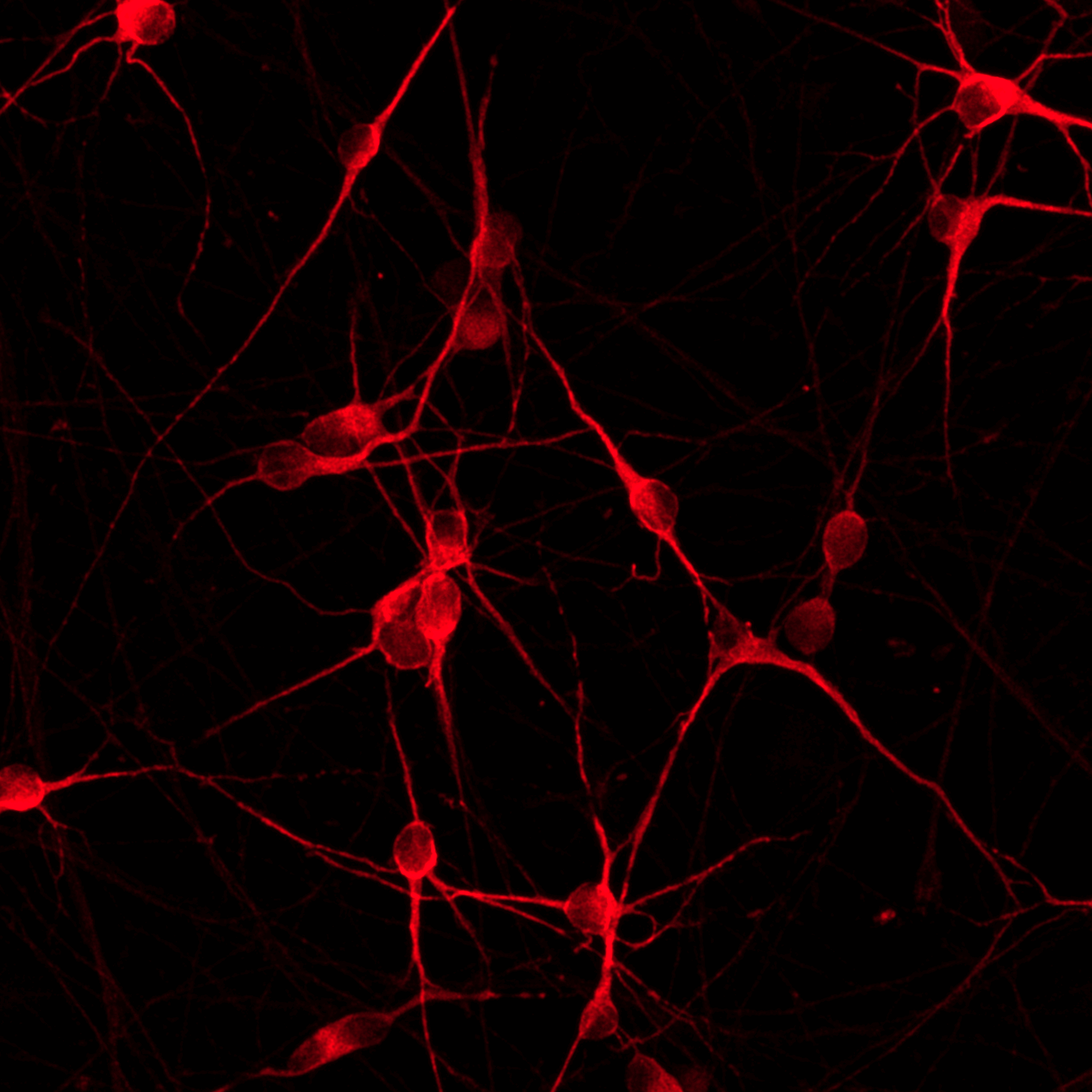
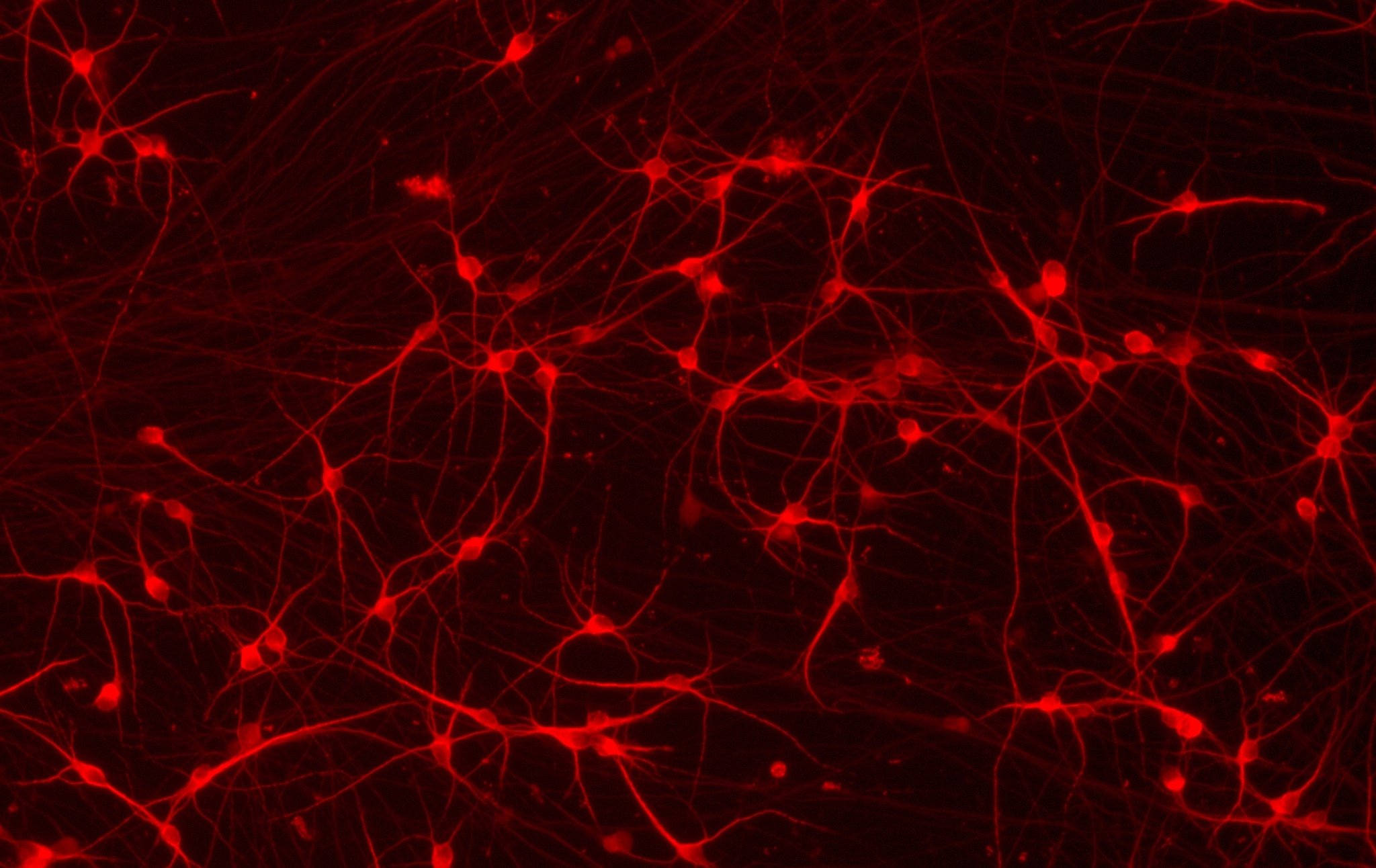
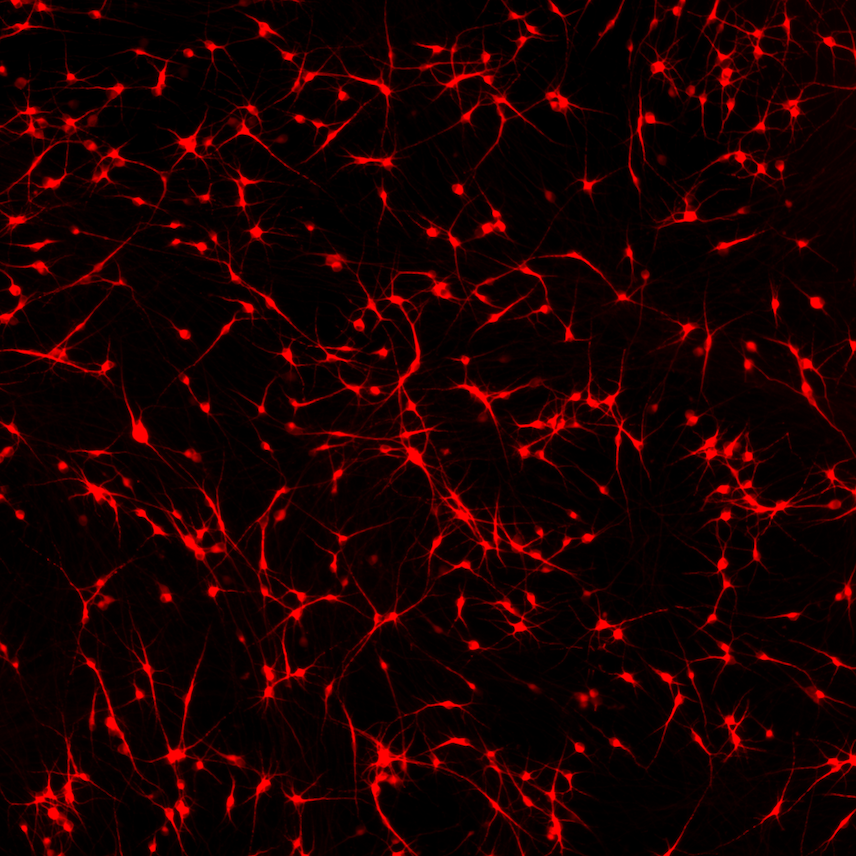
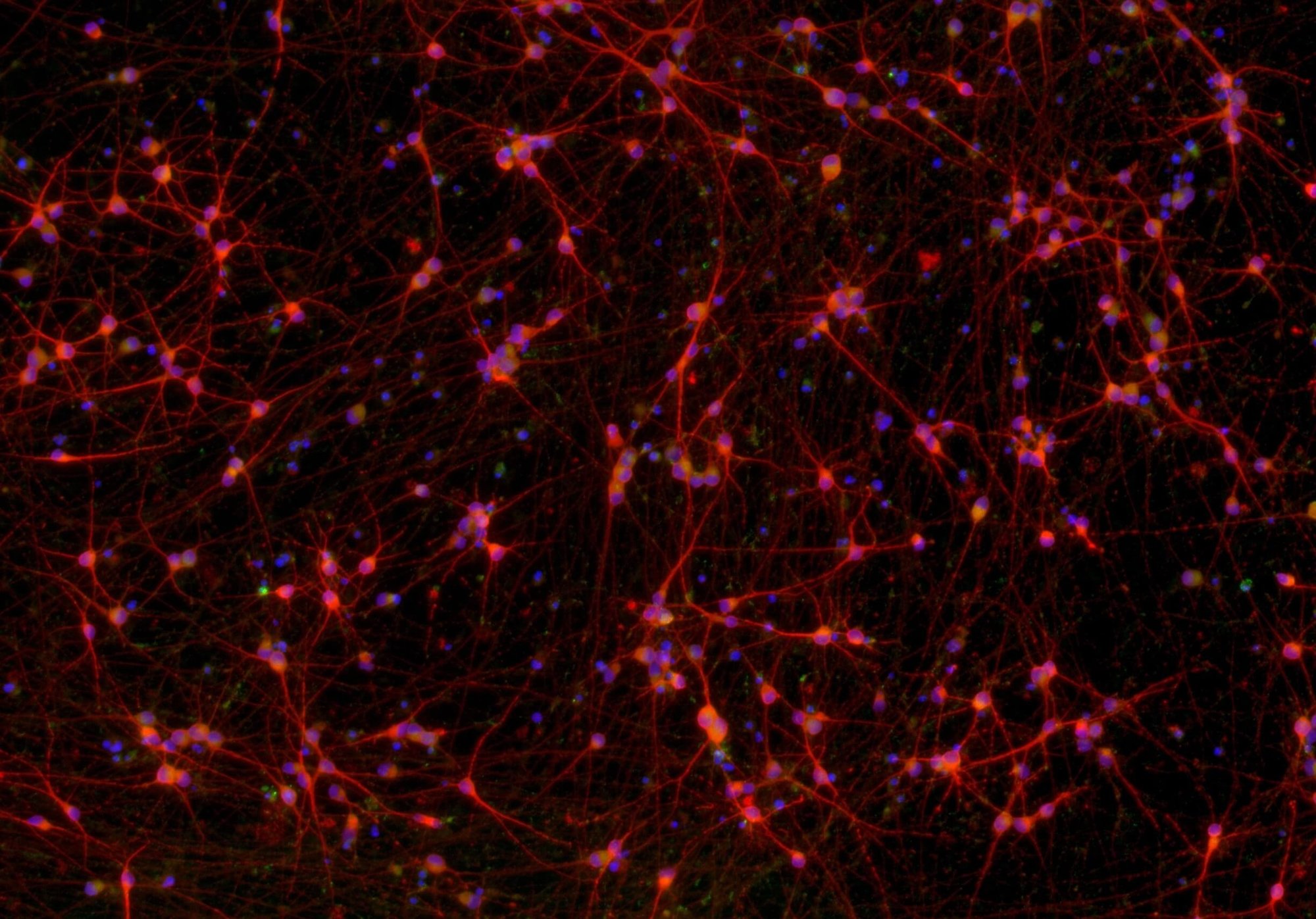
ioGlutamatergic Neurons
MAPT N279K/N279K
Human iPSC-derived FTD disease model
Place your order
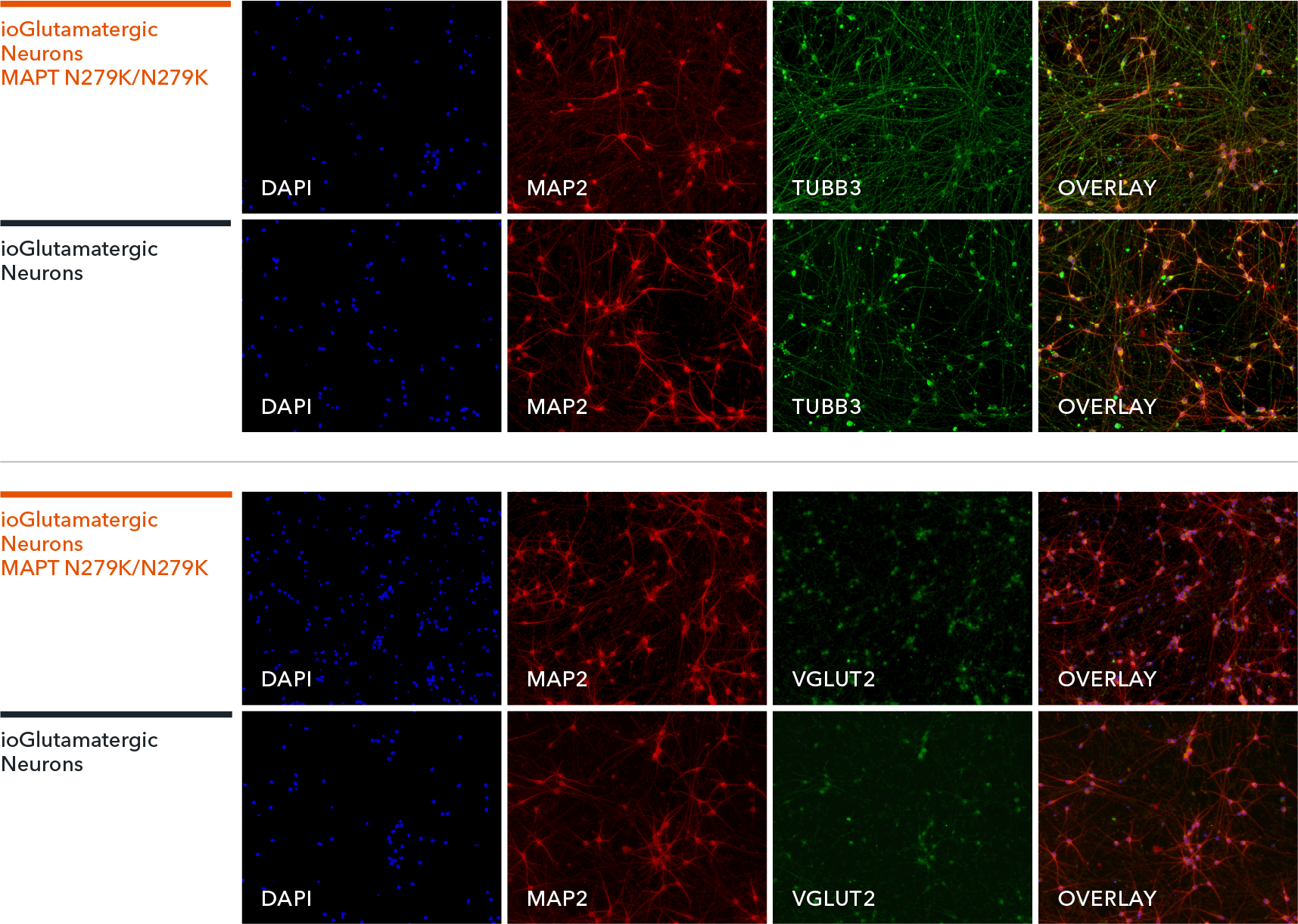
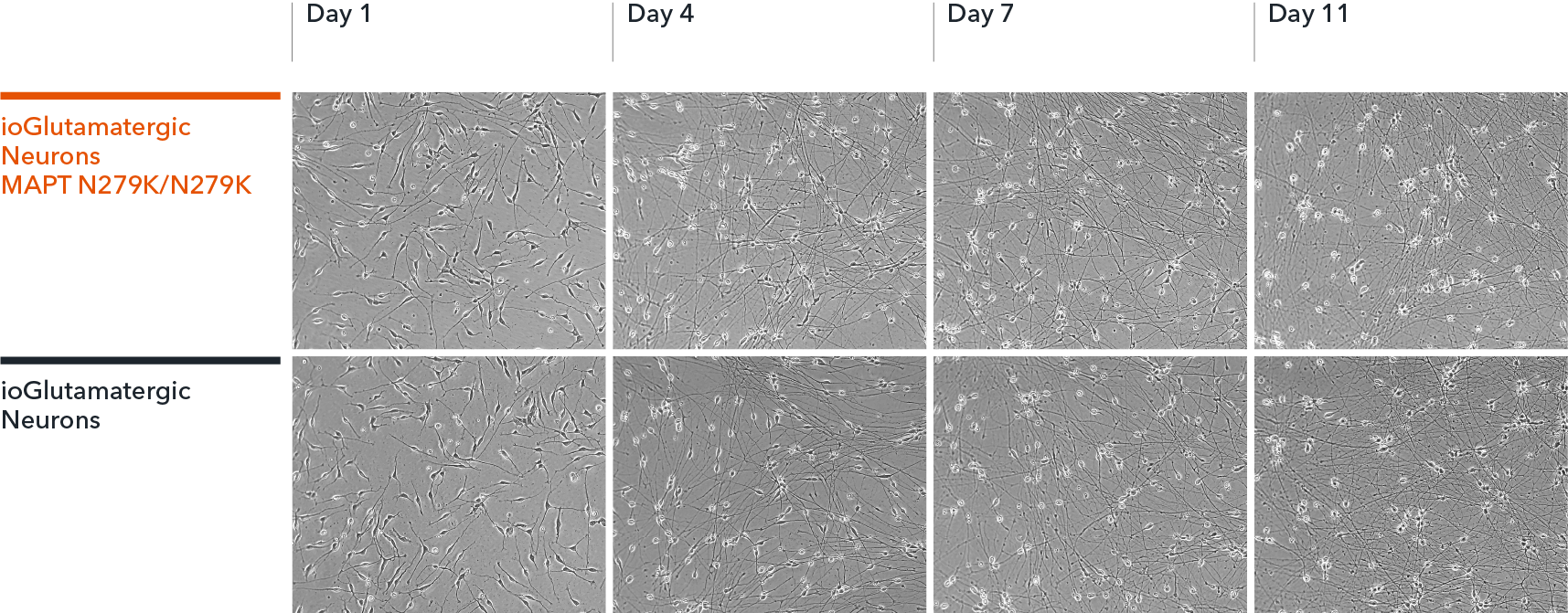
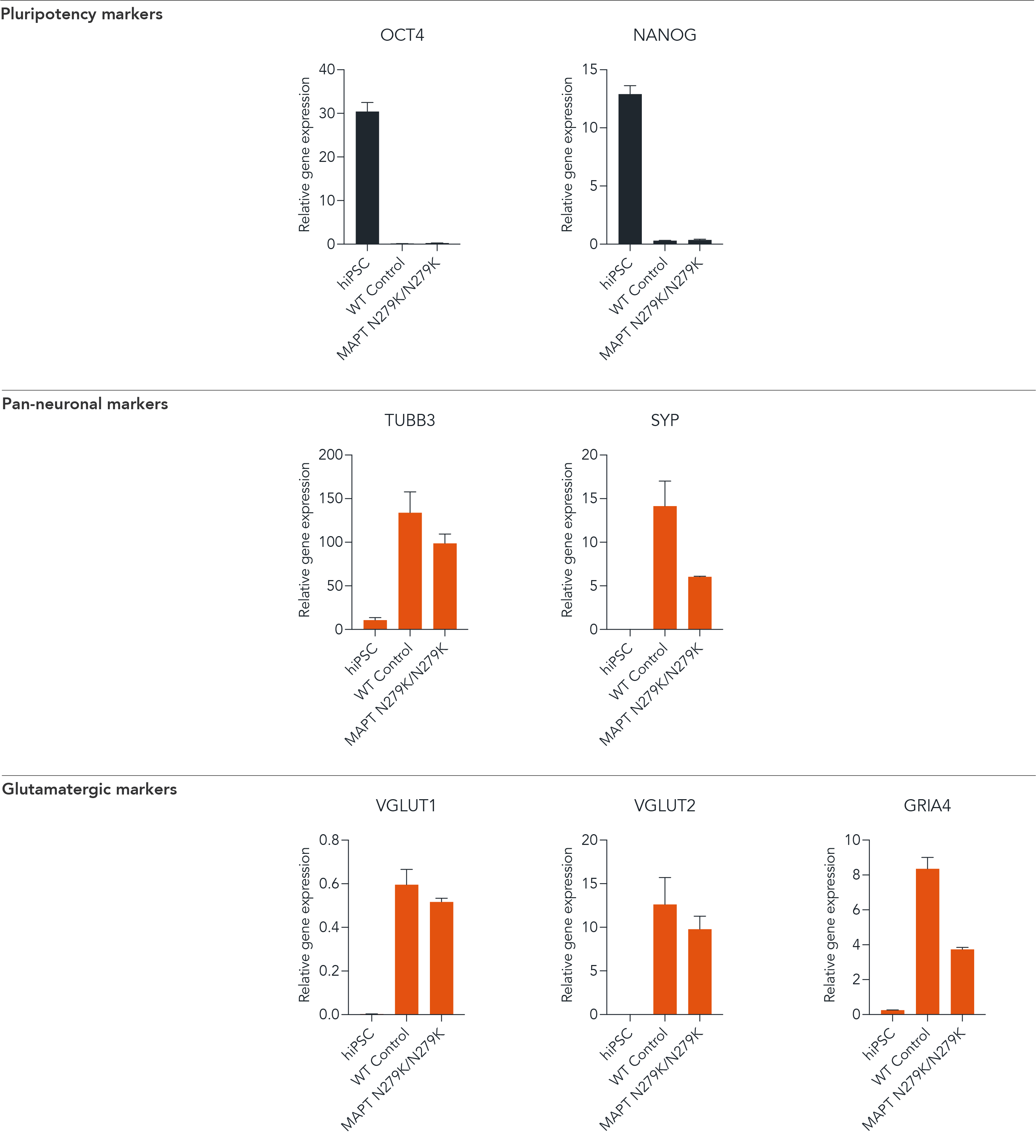
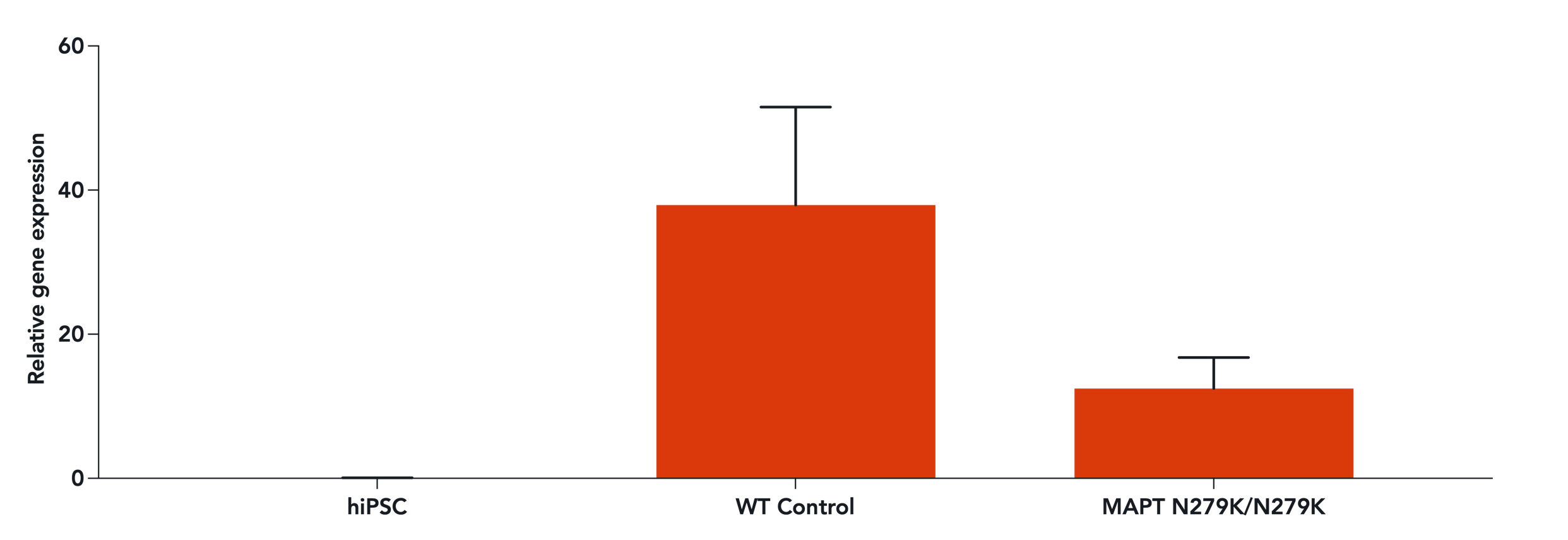
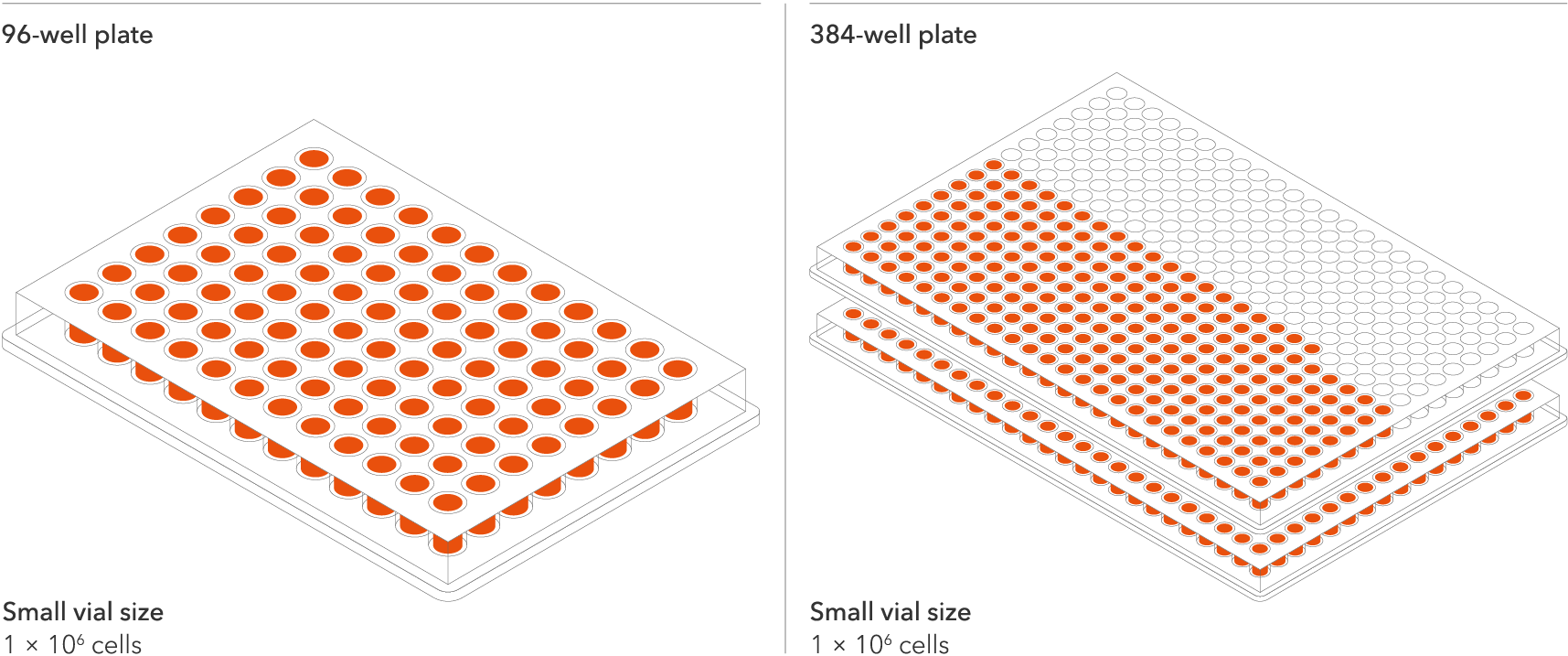
Confidently investigate your phenotype of interest across multiple clones with our disease model clone panel. Detailed characterisation data (below) and bulk RNA sequencing data (upon request) help you select specific clones if required.
per vial
A maximum number of 20 vials applies. If you would like to order more than 20 vials, please contact us at orders@bit.bio.
For academic discounts or bulk pricing inquiries, contact us




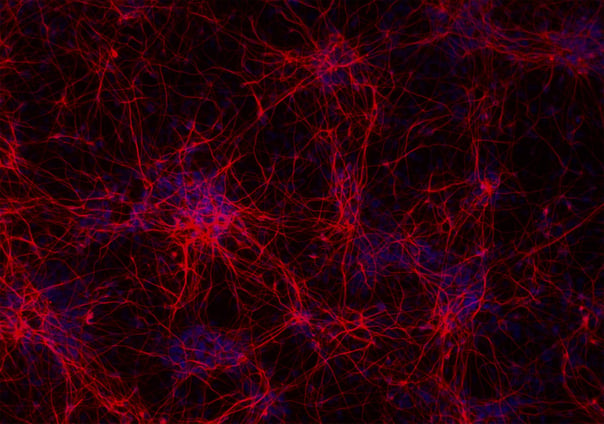

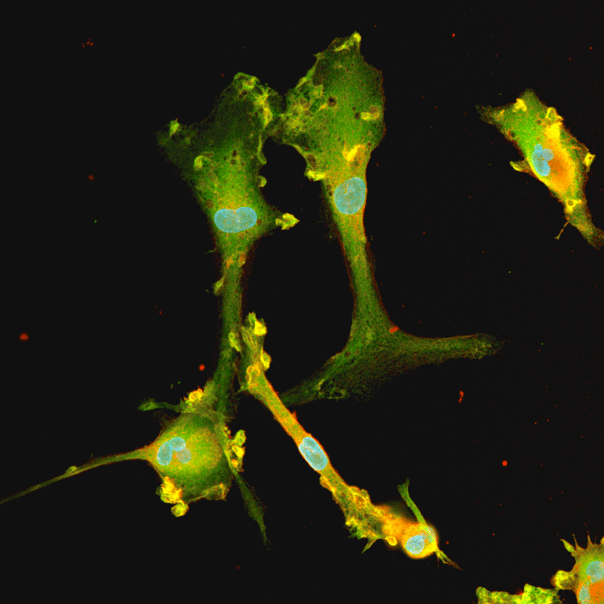

.png?width=1860&height=1260&name=bit.bio_3x2_ioGlutamatergic%20Neurons_MAP2_Hoescht_x20_hi.res%20(1).png)