10.10.2023 | Published by bit.bio
Microglia are macrophages, an immune cell type associated with the central nervous system (CNS). As dynamic cells, they’re sensitive to subtle alterations in their surrounding conditions such as the presence of nearby cells and debris. With a range of responses to environmental stimuli and roles in the body, an association between microglia dysfunction and CNS disorders has been emerging.1
At bit.bio, biologists Dr Camilla Fairbairn, from Cell Type Development and Hannah Garnett, from Manufacturing have worked with the team to develop human iPSC-derived ioMicroglia to provide scientists with functional, consistent, and highly-defined microglia at scale: “Microglia’s dynamic nature and role in immunity make them fascinating in terms of the assays they’re used in, the way they move and act, which make them such an interesting cell type to work with,” says Hannah. Camilla adds: “I’m interested in the different polarisation states of myeloid cells, how they can impact tumour formation, and their use in drug discovery and research into critical neurodegenerative diseases such as Alzheimer’s disease. Microglia are so fascinating because they have multiple phenotypes!”
In this blog, Camilla and Hannah share their five top tips for working with this cell type. Through gentle handling, partial media changes and using the optimal seeding density, along with embracing the dynamic morphology of microglia, it is possible to enhance the success of your microglia cultures.
Go straight to the top tips!
- Top tip #1: Microglia are dynamic cells, so expect to see different morphologies in your culture
- Top tip #2: Carry out partial media changes to avoid stressing the microglia
- Top tip #3: Use the correct seeding density for healthier microglia
- Top tip #4: Be gentle when handling microglia, to prevent shocking cells
- Top tip #5: Use hybrid media when co-culturing cells to optimise performance
What are microglia?
Microglia are the main immune cell type found in the brain, making up 10-15% of total cells within the CNS and their role involves immune defence and maintenance2. As part of this, microglia work in the body to survey neuronal function, play roles in neurogenesis and synaptic ‘pruning’, and are the first responders to infection,3 existing in multiple polarisation states depending on various factors and CNS environmental cues4. Microglia produce cytokines or phagocytose foreign bodies in response to immune stimuli and also play a key role in developing and modelling the CNS.
Camilla highlights that the impact of microglia in health and disease can be dependent on their polarisation state: “Microglia have two main roles in the body: defence and maintenance. They can be triggered to be pro-inflammatory and induce neuro-toxicity when under a sustained inflammatory response (classical, M1 phenotype) or can have a different polarisation state associated with development and neuro-protectivity (alternative, M2 phenotype). A continuum exists between the two states5, and dysregulation has been implicated in neurodegenerative disease.”
To date, Camilla says, the life sciences sector relies predominantly on rodent models to mimic disease states for drug discovery even though they do not always recapitulate human cell and disease phenotypes.“ To bridge what has so far been a ‘translational gap’, several in vitro human models have been developed for the study of microglia. Most typically, primary microglia are extracted directly from either embryonic, neonatal or adult tissue. However, primary human cells are limited in supply, difficult to source, and often show donor-to-donor and user variability.” That’s why, at bit.bio, she’s worked to develop precision reprogrammed hiPSC-derived ioMicroglia, which rapidly convert from hiPSCs to functional microglia in 10 days. “By consistently recapitulating human microglia function, with the ability to model brain complexity, ioMicroglia are helping scientists get to their data faster,” Hannah adds.
Top tip #1: Microglia are dynamic cells, so expect to see different morphologies in your culture
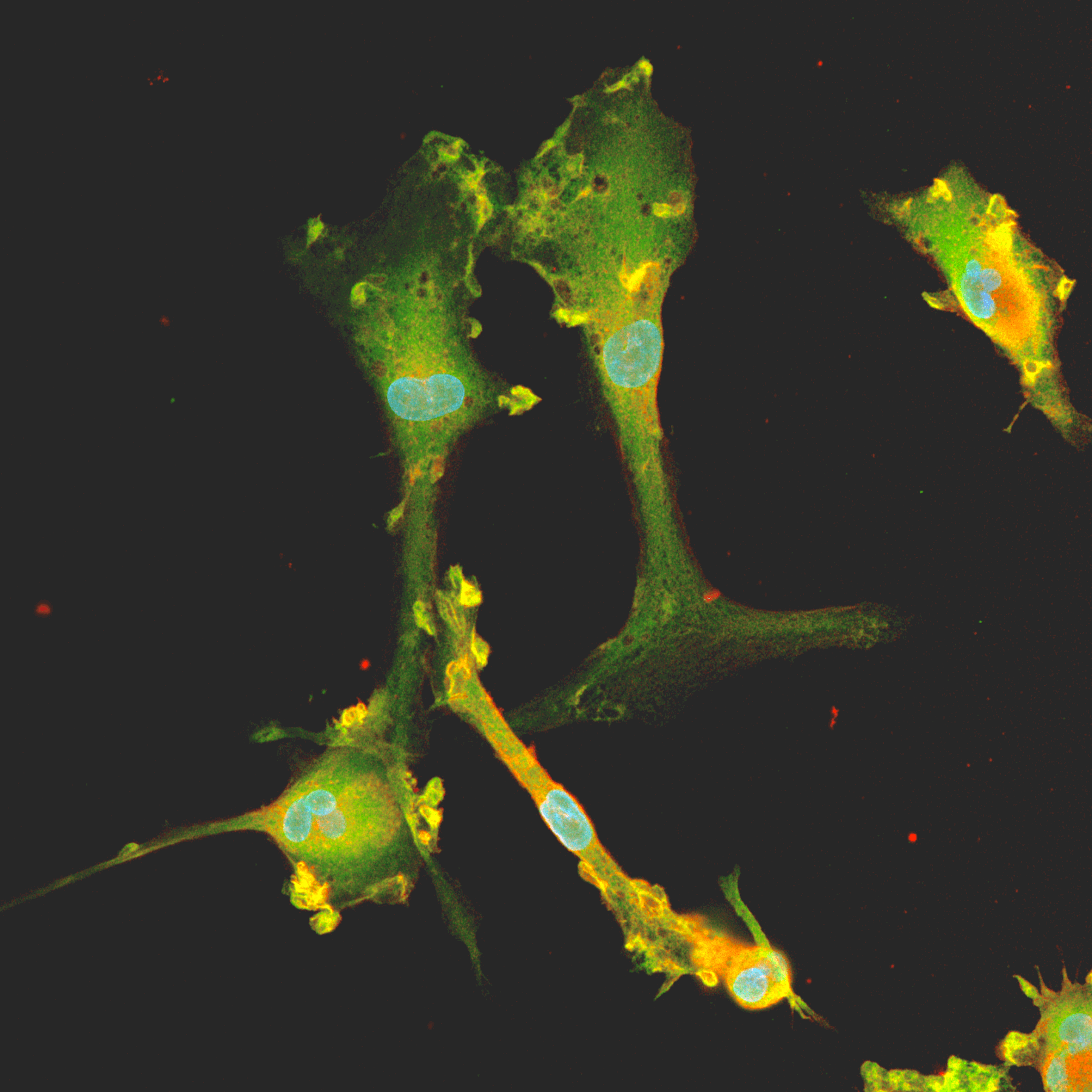
Since microglia constantly sense the microenvironment, they display diverse morphological and functional states.4 "It’s surprising when you first look at them, as they do change quickly. Leaving a cell culture flask on the benchtop for even a few minutes can easily make microglia change their morphology. That’s because this cell type is particularly sensitive to changes in their microenvironment,” says Hannah. Given their dynamism, microglia can quickly switch between ramified and ameboid morphologies so “don’t be surprised if the microglial cell culture looks different, particularly after media changes.”
Two observable morphologies include resting cells that have long and outstretched ramified processes, and activated ameboid cells that lack cellular processes (Fig. 1). The latter morphology looks round, with fluffy borders similar to a ‘fried egg’6. Microglia can also respond to bits of debris or protein filaments in the dish. In these instances, cells migrate to the debris and become activated, Hannah says, which can sometimes look a bit like a “zombie apocalypse” under the microscope. “The microglia are still healthy and functional, so don’t panic! If dead cells are present in the media, however, this could result from rough culture handling. This should be avoided as much as possible.”
Top tip #2: Carry out partial media changes to avoid stressing the microglia
Camilla recommends always leaving a small amount of media in the culture well when replacing media “because microglia are sensitive to changes, especially during the first few days after thawing. After the initial 24 hours post-thaw, while the cells are still stabilising, leave 10% of the media behind at the first media change to protect against stress.” When culturing microglia from day 4 onwards, Camilla recommends half-media changes to not stress the cells. This is because the balance and concentration of media components such as cytokines can impact the microglia’s changing states, and it’s good to minimise disruptions to that balance.
“If you accidentally change all of the media, then make sure to dilute components in the media, such as cytokines, as too high levels of cytokines can also negatively impact the culture.”
Top tip #3: Use the correct seeding density for healthier microglia
“We recommend a seeding density of 39,500 cells/cm2. If the density is higher, it can trigger microglia to respond by phagocytosing the cells around them, and if the density is lower, this can negatively affect their survival,” agree Hannah and Camilla.
Hannah jokingly refers to microglia as “introverted extroverts”. Underseed these cells, and they’ll become stressed as a result of insufficient contact with each other. Overseed them, and the microglia will begin to phagocytose one another to maintain homeostasis in the culture. Camilla says this observation is unique to hiPSC-derived microglia in vitro, as other macrophage subtypes, such as monocyte-derived macrophages, can achieve much higher densities. This is why bit.bio’s protocol specifies optimised seeding densities, to maintain stable microglia cell culture and achieve reliable, functional readouts.
Top tip #4: Be gentle when handling microglia, to prevent shocking cells
Microglia don’t fully adhere to normal cell culture surfaces as they are motile cells, so when handling these cell cultures, be gentle. If you're using an automated or electronic pipette (like an Integra, for example), use it at a slow speed, 1 mL over 20 seconds - this is equivalent to ‘speed 1’ on the Integra Voyageur pipette. Alternatively, when using a P1000 manual pipette, ensure you're very slow in pipetting media in and out of the plate, and always add or remove media from the side of the plate. For optimal attachment, coat the cell culture plate with poly-L-lysine.
When preparing thawed cells, it’s useful to split the basal media into two: one made up to complete media and placed in a water bath (used for seeding), and the other kept at room temperature for thawing.
Any temperature over 21°C can generally cause shock to thawing cells. “I wait until there’s a tiny ice crystal left in the cryovial before moving the suspension into a 15 mL tube, and then add room temperature microglia basal medium (b:M medium) media,” advises Hannah.
Top tip #5: Use hybrid media when co-culturing cells to optimise performance
Camilla has tested a few different, effective protocols for the co-culture of microglia cells with neurons. One approach is to culture bit.bio’s human iPSC-derived ioGlutamatergic Neurons and ioMicroglia in parallel monocultures up to day 10 post-thaw and then combining the microglia with the neurons in a co-culture.
Alternatively, ioGlutamatergic Neurons can be cultured to day 10 post-thaw, while ioMicroglia can be thawed and cultured for 24 hours before being combined with the neurons.
Co-culturing microglia with neurons helps microglia cells mature, leading to a more pronounced phenotype7, with ioMicroglia displaying a more ramified morphology and developing interactions with the neurons3 (Fig. 2).
When co-culturing microglia with neurons, do remember to use a hybrid media to cater to both cell types, like the one described in our co-culture protocol. This includes both complete microglia maturation and maintenance medium as well as glutamatergic neuron medium (see User Manuals for more details).
Figure 2. Video showing cryopreserved ioMicroglia and ioGlutamatergic Neurons from bit.bio in co-culture. ioGlutamatergic Neurons were thawed and cultured independently for 10 days, while the ioMicroglia were separately seeded and cultured from D0 to D1 (24 hours), detached and then added directly to D10 ioGlutamatergic Neurons. The cells were co-cultured for 6 days before imaging. Images were captured once every 6 min 24 secs for 3 h 31 min using Nanolive’s automated imaging platform. ioMicroglia demonstrate the ability to form a stable co-culture with ioGlutamatergic Neurons.
Summary
Camilla and Hannah are confident that any scientist, whether a beginner or seasoned expert in cell culture, should be able to get the hang of microglia cell culture by following their guidance above. This includes being mindful of the dynamic phenotype of microglia, leaving some of the original media remaining during media changes, using the correct seeding density, being gentle when handling the cells, and making sure to use hybrid media that supports both microglia and neurons during co-culture experiments. With these five tips in mind, you should be well on your way to successfully culturing microglia and getting high-quality functional readouts!
Do you have any cell culture tips to share? We’d love to hear from you!
Experts in neuroimmunology and neurodegeneration across academia and industry have successfully incorporated our ioMicroglia into functional assays that support their drug discovery workflows and disease research. Multiple independent labs have shown ioMicroglia exhibit a pro-inflammatory response to amyloid beta and LPS, which can be inhibited with dexamethasone. Dive into the cytokine secretion datasets here.
About Dr Camilla Fairbairn and Hannah Garnett
“Dr Camilla Fairbairn is a Team Lead in bit.bio’s Cell Type Development Team, where she is focused on Immunology. She has a PhD in pathology from the University of Cambridge, where she studied an immune-related cancer, a T-cell lymphoma, and how it arises. She has experience with therapeutic antibodies and small molecules in the T-cell space in immuno-oncology, including rescuing T-cell dysfunction and trying to reverse immunosuppressive phenotypes in myeloid cells.”
Hannah Garnett is a Scientist at bit.bio, working in the Manufacturing of microglia. She has an MSc from the University of Suffolk in Regenerative Medicine. Hannah has experience in iPSC-derived manufacturing facilities investigating classical cell differentiation pathways and generating iPSCs. She is part of bit.bio’s manufacturing development team, ensuring cells are fully scalable as well as carrying out quality control.
 Camilla FairbairnTeam Leader, bit.bio
Camilla FairbairnTeam Leader, bit.bio Hannah GarnettScientist, bit.bio
Hannah GarnettScientist, bit.bio
References
1. Prinz M, Masuda T, Wheeler MA, et al. Microglia and central nervous system–associated macrophages—from origin to disease modulation. Annual review of immunology. 2021;39:251-277.
https://doi.org/10.1146/annurev-immunol-093019-110159
2. Masuda T, Sankowski R, Staszewski O, et al. Microglia heterogeneity in the single-cell era. Cell reports. 2020;30(5):1271-1281. https://doi.org/10.1016/j.celrep.2020.01.010
3. Raman M, et al. Rapid and consistent generation of functional microglia from reprogrammed hiPSCs to study mechanisms in neurodegeneration and neuroinflammation. Poster at SfN 2022. 11-15 November, Washington D.C.
4. Paolicelli RC, Sierra A, Stevens B, et al. Microglia states and nomenclature: A field at its crossroads. Neuron. 2022;110(21):3458-3483. https://doi.org/10.1016/j.neuron.2022.10.020.
5. Guo S, Wang H and Yin Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022;14:815347. https://doi.org/10.3389/fnagi.2022.815347.
6. Martinez A, Hériché JK, Calvo M. Characterization of microglia behaviour in healthy and pathological conditions with image analysis tools. Open Biol. 2023 13(1):220200. https://doi.org/10.1098/rsob.220200.
7. Haenseler W, Sansom SN, Buchrieser J, et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Reports. 2017 Jun 6;8(6):1727-1742. https://doi.org/10.1016/j.stemcr.2017.05.017.