28.04.2023 | Published by bit.bio
Standing before a room of prominent psychologists in 1906, a 42-year-old scientist named Alois Alzheimer described a “peculiar severe disease process of the cerebral cortex” that had afflicted one of his patients. Far from evoking the awe he expected, Alzheimer’s lecture barely caused a stir among the audience and was little more than a footnote in subsequent retellings of the day’s proceedings1. Despite its reception, the disease he described, what we now know as Alzheimer’s disease, would grow to be a household name. One known for its devastating impact on patients and their families.
More than 50 million people are affected by Alzheimer’s disease worldwide, with many more projected in the years to come2. Over the last century, progress towards understanding and treating the disease has been limited. We know multiple forms of the disease exist, some driven by clear genetic factors, and others that are far more complex. Regardless of the disease subtype, neuroinflammation has recently been shown to be a critical process that, paradoxically, appears to be both a protective and pathological force in the disease3.
Naturally, Alzheimer’s disease research over the past 10 years has placed an emphasis on studying the behaviour of a particularly inflammatory cell type: microglia. These brain resident immune cells carry out a range of functions, including synaptic remodelling and neuroprotection. In this latter capacity, microglia respond to injury and disease stimuli by releasing inflammatory cytokines, reactive oxygen species, and other signals that drive an inflammatory immune response4.

A photograph of Auguste Deter taken in 1901. Auguste was the first patient of Alois Alzheimer to be described with Alzheimer's Disease. Image available under a public domain license on wikicommons.
“This inflammatory response is a very beneficial function of microglia in the early stages of Alzheimer's disease,” explains Dr Matthias Pawlowski, a board-certified clinical neurologist who heads the Dementia Sensitive Hospital within the Germany-based University Hospital Munster. Pawlowski is leading several projects aimed at better understanding the pathology of Alzheimer’s disease and, ultimately, developing novel therapeutic strategies.
In a recent webinar describing the role of microglia in Alzheimer’s disease pathogenesis, Pawlowski explained that “during later stages of the disease, the activating stimuli, such as amyloid beta plaques, cannot be completely cleared, and thus lead to chronic activation of the microglia.” Chronic microglia activation in Alzheimer’s disease, it seems, leads to prolonged inflammatory signalling, tissue damage, and a detrimental feedback loop that drives neurodegeneration. Stopping this damaging cycle has become the focus of many drug development efforts4.
Modelling microglia cells in vitro to better understand Alzheimer’s disease
Less than a year after Alois Alzheimer’s seminal lecture, Ross Granville Harrison, then an associate professor of anatomy at Johns Hopkins University, reported the first instance in which individual cells from a living organism (in this case, a frog) were kept alive in a dish for an extended period of time5. This moment marked the very beginning of conventional in vitro cell culture and kicked off a century of incredible scientific achievement. With cells grown in a dish, researchers could study the behaviour and molecular biology of specific cell types, they could model aspects of disease in a controlled setting, and they could rapidly screen potential therapeutics on a large scale.
Put simply, having the ability to culture cells in vitro is foundational to the study and eventual treatment of many diseases. However, not all cell types are amenable to in vitro culture. Microglia cells, for example, are particularly challenging because they are non-proliferative in their mature state. This means that you only get what you collect when you harvest microglia from a patient or cadaver. With a short supply of non-renewing cells, very few experiments can be performed, and this greatly limits the pace of research on microglia6.
To overcome these hurdles, researchers have turned to using both immortalised microglia cell lines and induced pluripotent stem cells (iPSC). The former are not ideal because, in gaining a high proliferative capacity, they are imperfect models of mature, non-proliferative microglia. Human iPSCs, though, can be coaxed into becoming iPSC-derived microglia cells through directed differentiation. With this approach, scientists have learned a great deal about the embryonic development of microglia and certain functions in disease pathology.
Limitations of current microglial models used in research and drug discovery
| Model | Limitations |
| Animal/Rodent Models |
|
| Primary Microglia (human/rodent) |
|
| Human/Rodent immortalised cell lines |
|
| hiPSC derived microglia (directed differentiation |
|
Despite this, for researchers like Pawlowski, who are studying the pathogenesis of Alzheimer’s, directed differentiation presents important drawbacks. In his recent webinar, Pawlowski explained the limitations he and others face: “From a technical viewpoint, there's still a lot of room for improvement. The problem with these protocols is that you need a very experienced researcher in stem cell research, and all of these protocols take quite a long time to complete. For example, the current gold standard protocol takes 38 days to differentiate the iPSC cells into more or less mature microglia-like cells.”
The complex and prolonged directed differentiation process is prone to user-dependent errors leading to batch-to-batch inconsistencies. When that happens, the cell generation process has to start over again, costing time and resources. And, when all is said and done, the final population of cells consists of a mixture of mature and partially differentiated cell types, thus requiring cell sorting to obtain a pure population of mature microglia. In short, directed differentiation is a labour and resource-intensive process. For these reasons, the directed differentiation of human pluripotent stem cells to microglia is not scalable. For a more in-depth exploration of this, read Speicher and Pawlowski’s 2019 review, wherein the various directed differentiation techniques are discussed (see Figure 1 in the paper for a good overview)6.
A scalable source of microglia would greatly accelerate Alzheimer’s disease research by enabling exploratory and high throughput research. For example, large studies could be carried out to analyse proteomic, transcriptomic, and epigenetic changes among microglia in response to specific developmental and disease stimuli, the results of which may reveal novel therapeutic targets and bring finer resolution to our understanding of disease pathology. Additionally, large-scale production of human microglia enables higher throughput drug screening in a more human-relevant model. By using cells that closely mimic human microglia in vivo, drug screening has a better chance of identifying beneficial therapeutic compounds.
Towards a defined and scalable in vitro cell culture model for Alzheimer’s research
Whereas directed differentiation of iPSC cells is limited in scalability and consistency, a new approach, optimised inducible overexpression (opti-ox), solves this problem.
opti-ox technology is an approach to transcription factor-mediated cellular reprogramming developed by Mark Kotter's laboratory at the University of Cambridge7 to rapidly and efficiently force iPS cells into a specific cellular identity. This is achieved by first identifying the essential combination of transcription factors (the cell identity code) that, when expressed in a cell, precisely activates the entire networks of genes that collectively define the cell’s behaviour and identity.
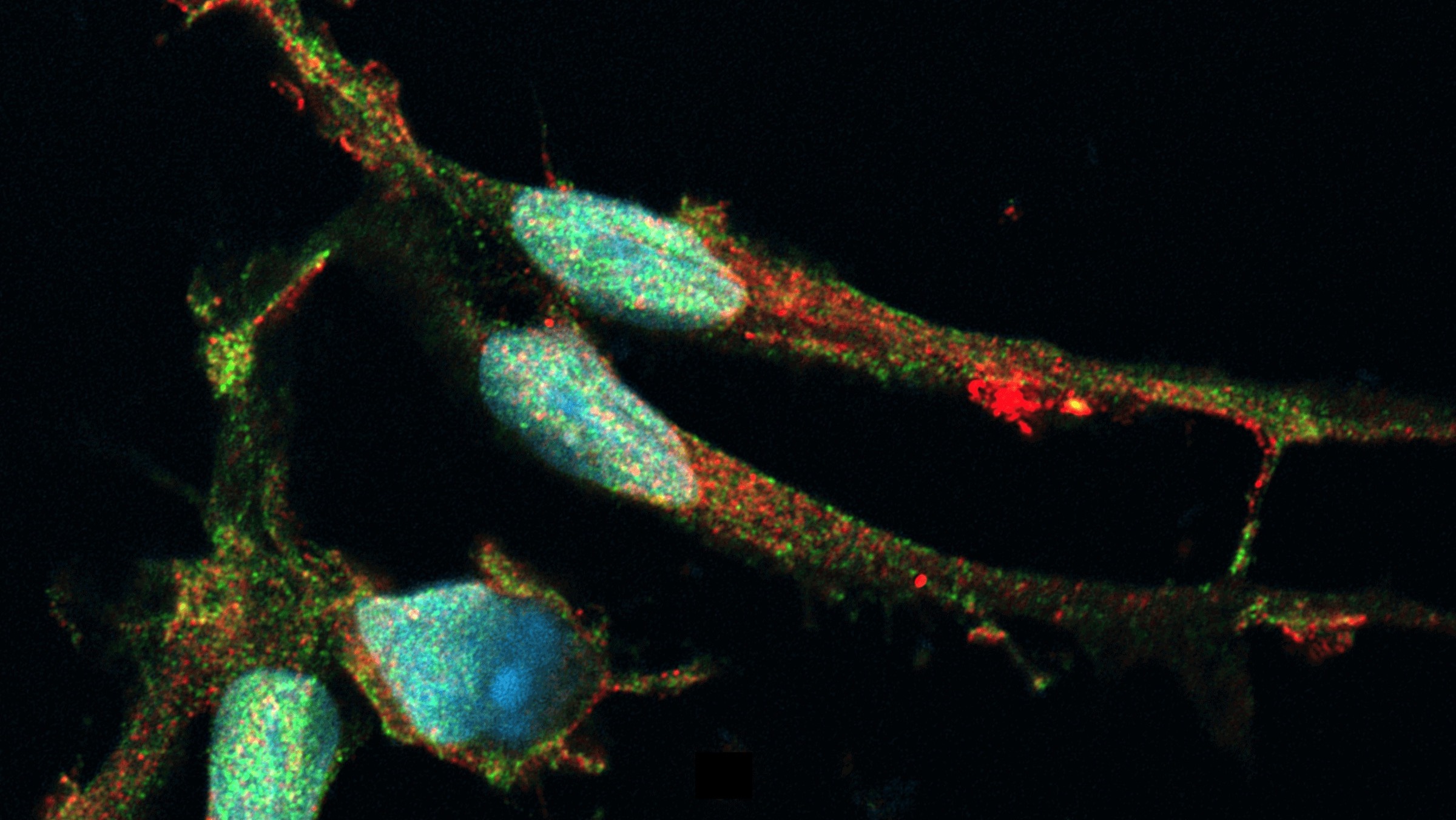
Immunofluorescent staining for microglial markers TMEM119 (red) and P2RY12 (green) in opti-ox powered human iPSC-derived microglia, counterstained with DAPI (blue).
Once identified, the genes coding for these identity-defining transcription factors are synthesized and placed into a genetic cassette that will serve as a delivery payload, enabling all of the genes in the cassette to be simultaneously inserted into the genome of an iPSC line.
However, these cassettes can’t simply be inserted anywhere in the genome—doing so can disrupt existing genes or, as often occurs, result in the exogenous DNA being silenced through epigenetic modification. Such silencing nullifies the expression of the transcription factors. The level of silencing also differs between each cell in the population, leading to poor consistency for downstream experiments as seen with existing reprogramming methods.
To ensure that the newly inserted genes are always turned on when needed, the opti-ox approach precisely places gene cassettes into genomic locations known as “safe harbours”. In these genomic regions, no other gene will be disrupted, and no silencing of the exogenous DNA will occur. Additionally, the cassettes contain doxycycline-inducible elements, meaning the genes in the cassette can be turned on at precise times, like flipping a light switch.
Collectively, the opti-ox approach allows researchers to achieve far more precise and efficient cellular reprogramming of iPSCs on a large scale. Or, as Pawlowski described in a 2017 article, opti-ox can be used “as an inexhaustible source for highly scalable, rapid, single-step, virus-free, chemically defined, fully reproducible, and deterministic generation of iPSC-derived cell lines.”7
In his recent webinar, Pawlowski partnered with bit.bio’s Senior Product Manager, Malathi Raman, PhD, who described the application of opti-ox technology to generate fully mature human microglia cells in just 10 days post-thaw. The fast and simple process helps open the door to larger-scale studies on human microglia and will likely quicken the pace of Alzheimer’s disease research. To this end, these cells can be used for microglia neuron co-culture, enabling more complex in vitro modelling of Alzheimer’s disease.
With such a defined and scalable source of microglia, researchers like Pawlowski can carry out larger-scale studies that may illuminate disease mechanisms and, hopefully, uncover better therapeutic options for the millions of people affected by Alzheimer’s disease.
Watch Pawlowski’s webinar to learn more about the emerging role of microglia in Alzheimer’s disease pathogenesis and how opti-ox technology may help accelerate research efforts.
References
- Hippius, Hanns, and Gabriele Neundörfer. “The discovery of Alzheimer's disease.” Dialogues in clinical neuroscience vol. 5,1 (2003): 101-8. doi:10.31887/DCNS.2003.5.1/hhippius
- GBD 2019 Dementia Forecasting Collaborators. “Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019.” The Lancet. Public health vol. 7,2 (2022): e105-e125.
- Heneka, Michael T et al. “Neuroinflammation in Alzheimer's disease.” The Lancet. Neurology vol. 14,4 (2015): 388-405. doi:10.1016/S1474-4422(15)70016-5.
- Hemonnot, Anne-Laure et al. “Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities.” Frontiers in aging neuroscience vol. 11 233. 30 Aug. 2019, doi:10.3389/fnagi.2019.00233
- Harrison, Rose G., et al. “Observations of the Living Developing Nerve Fiber.” The Anatomical Record, vol. 1, no. 5, 1 June 1907, pp. 116–128, 10.1002/ar.1090010503.
- Speicher, Anna M et al. “Generating microglia from human pluripotent stem cells: novel in vitro models for the study of neurodegeneration.” Molecular neurodegeneration vol. 14,1 46. 19 Dec. 2019, doi:10.1186/s13024-019-0347-z
- Pawlowski, Matthias et al. “Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes.” Stem cell reports vol. 8,4 (2017): 803-812. doi:10.1016/j.stemcr.2017.02.016